Abstract
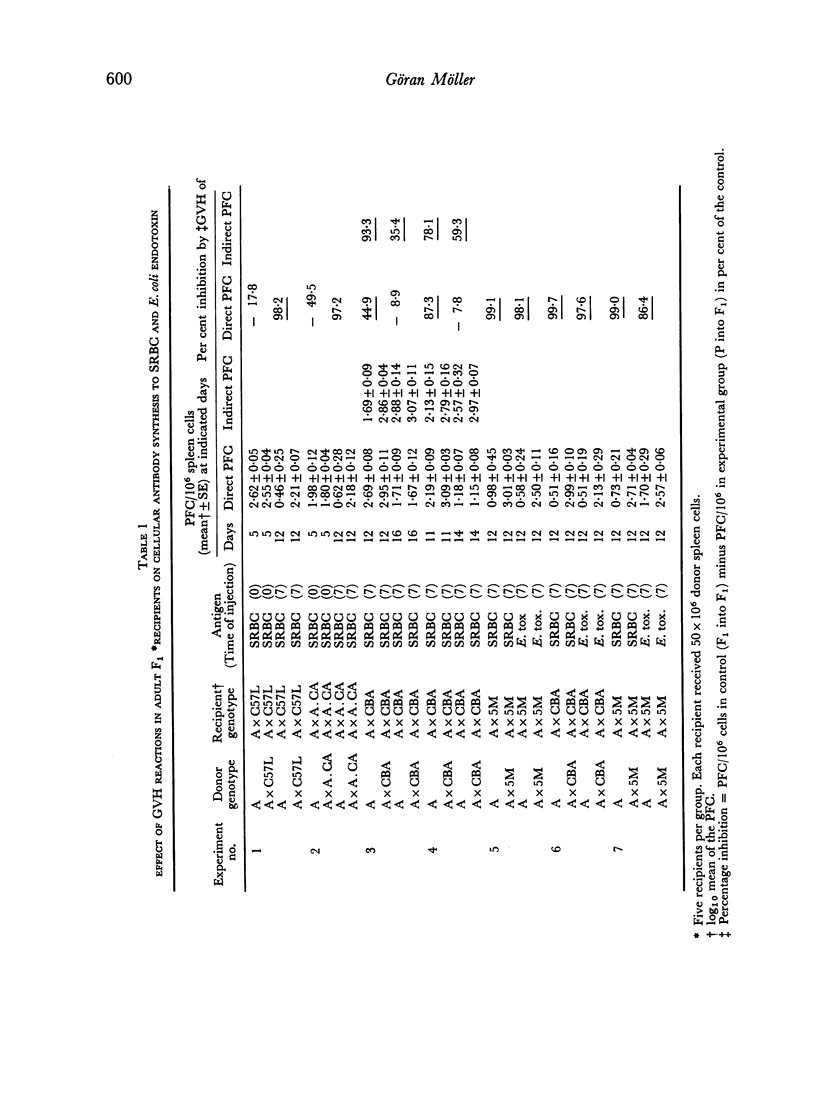
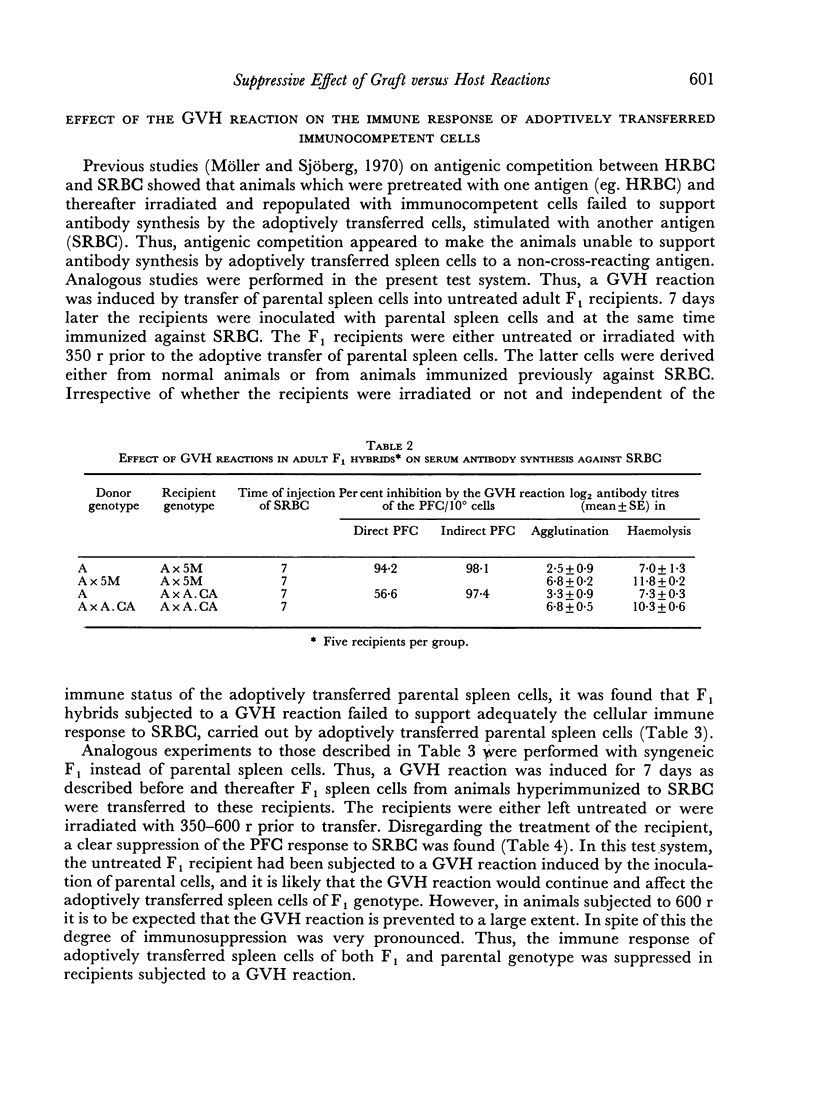
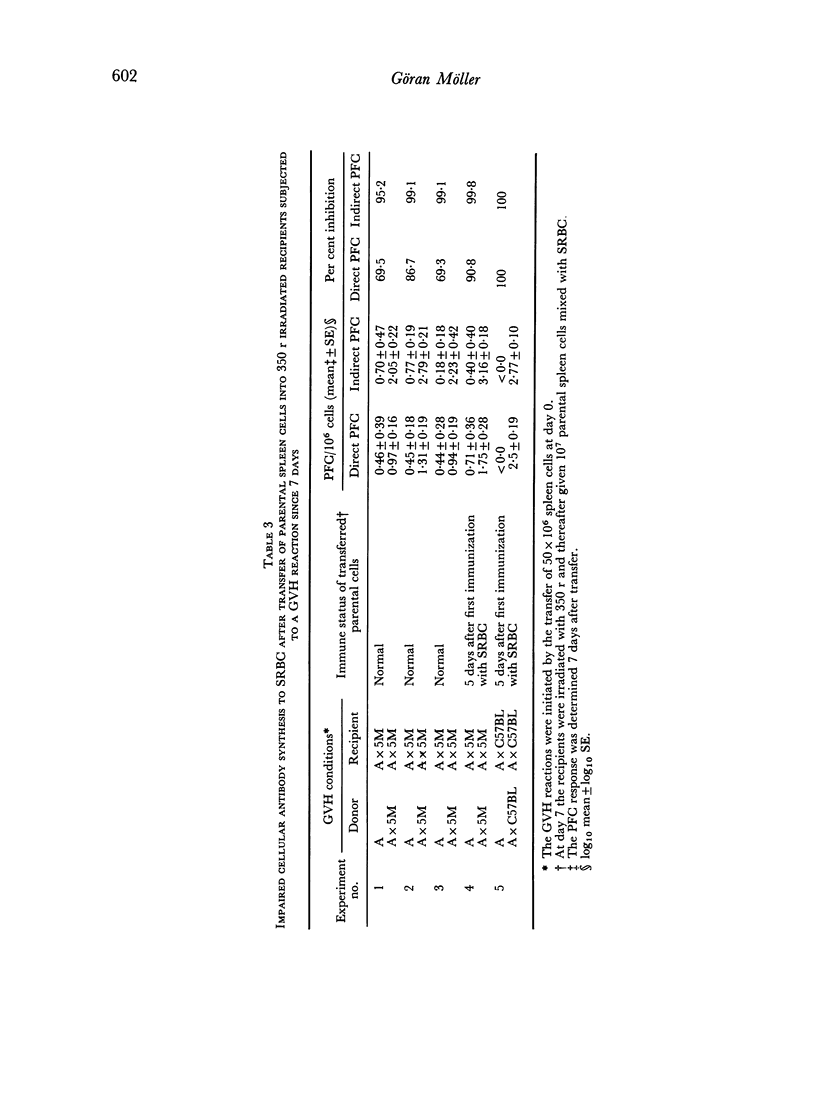
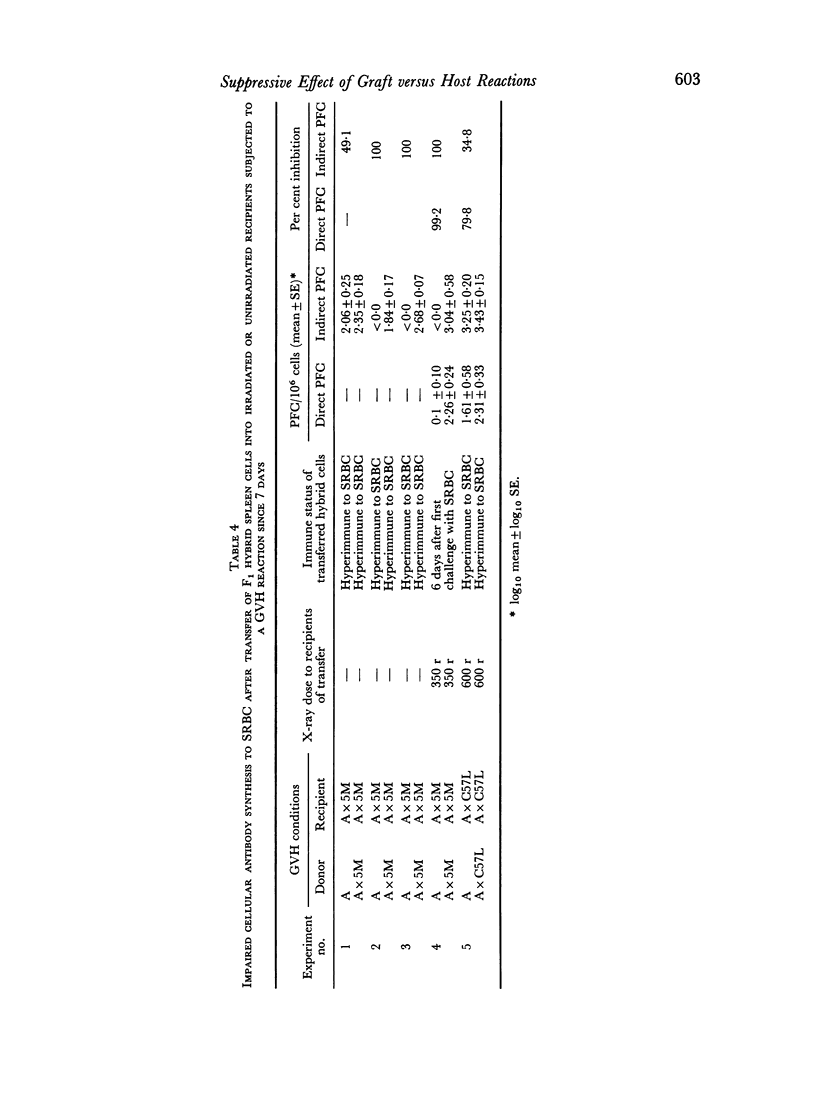
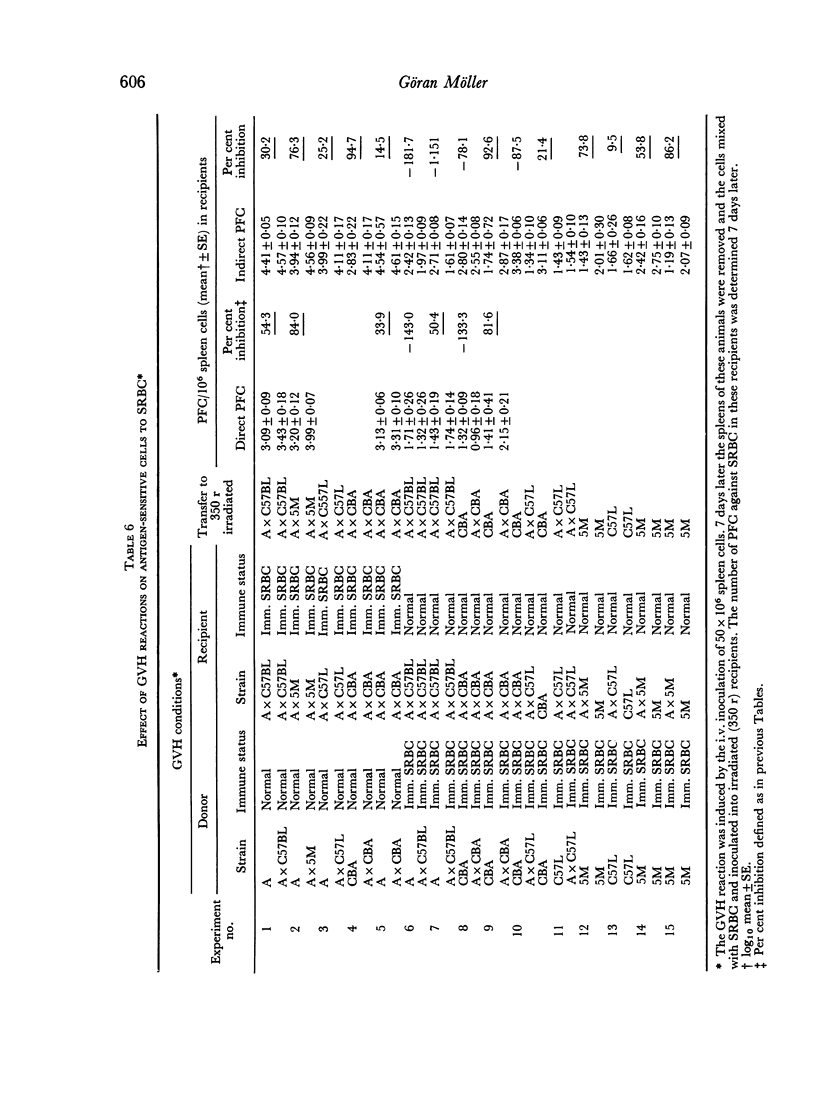
Graft versus host (GVH) reactions induced by the inoculation of parental spleen cells into adult untreated F1 recipients caused a marked suppression of the cellular and humoral immune response to sheep red cells (SRBC) and Escherichia coli lipopolysaccharide, provided the GVH reaction was induced 7 days before immunization with SRBC. Adoptive transfer of parental or F1 spleen cells mixed with SRBC into irradiated F1 recipients, which had been subjected to a GVH reaction for 7 days, resulted in marked suppression of cellular antibody synthesis to both antigens.
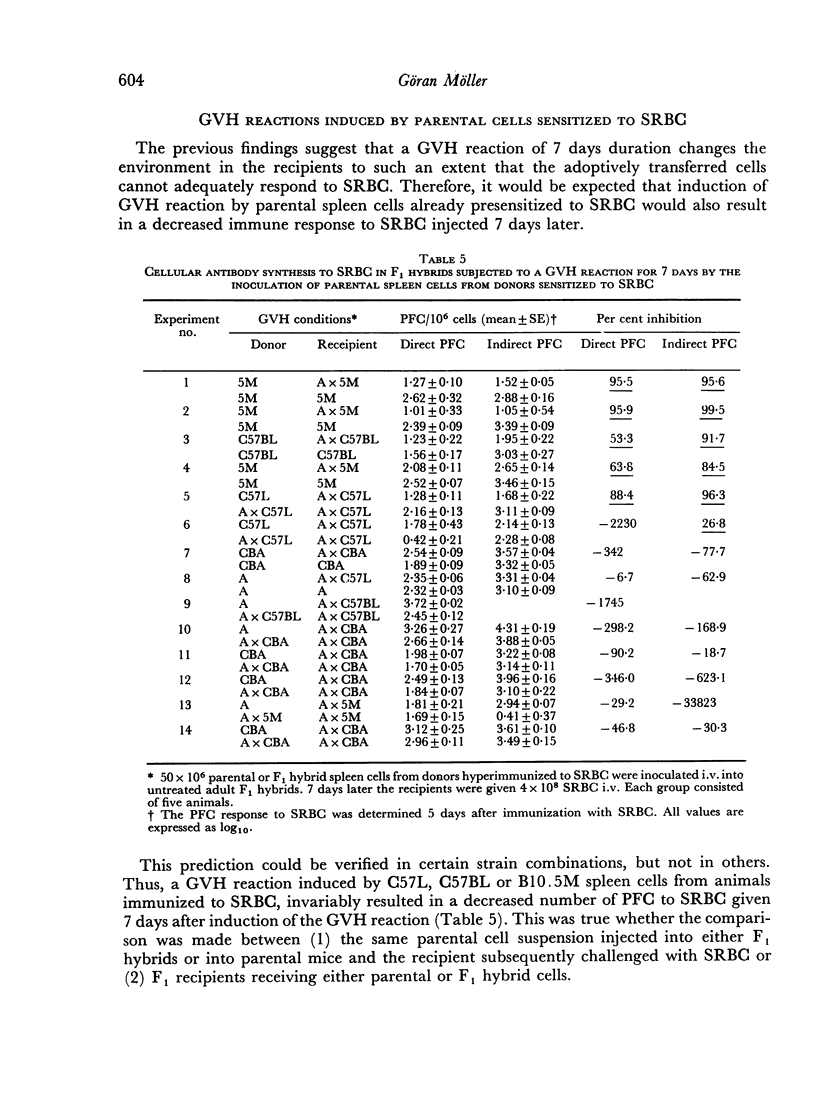
When the GVH reaction was induced by parental spleen cells from donors immunized to SRBC and the immune response to SRBC tested after 7 days, a marked suppression of cellular antibody synthesis occurred when the parental cells were of H-2b genotype, but not if they were of H-2a or H-2k genotypes.
The number of antigen-sensitive cells of parental genotype sensitive to SRBC in animals being subjected to a GVH reaction for 7 days was unaffected when the donors were of H-2a or H-2k genotypes, but decreased when the donors were H-2b. The number of antigen-sensitive cells of F1 genotype was only slightly decreased by a GVH reaction.
It is suggested that the suppressive effect of a GVH reaction on antibody synthesis to other antigens represents an example of antigenic competition. This phenomenon would be caused by suppressed proliferation of immunocompetent cells of bone-marrow origin and not by competition for pluripotent antigen-sensitive cells. This suppression would be mediated by antigen stimulated thymus-derived lymphocytes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYSE E. A. The fate of mouse spleen cells transplanted into homologous and F1 hybrid hosts. Immunology. 1959 Apr;2(2):170–181. [PMC free article] [PubMed] [Google Scholar]
- Brody N. I., Siskind G. W. Studies on antigenic competition. J Exp Med. 1969 Oct 1;130(4):821–832. doi: 10.1084/jem.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELADA F., WELSHONS W. J. Demonstration of F1 hybrid anti-parent immunological reaction. Proc Natl Acad Sci U S A. 1962 Mar 15;48:326–331. doi: 10.1073/pnas.48.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUDKOWICZ G., STIMPFLING J. H. DEFICIENT GROWTH OF C57BL MARROW CELLS TRANSPLANTED IN F1 HYBRID MICE. ASSOCIATION WITH THE HISTOCOMPATIBILITY-2 LOCUS. Immunology. 1964 May;7:291–306. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Siminovitch L., Till J. E., McCulloch E. A. A transplantation assay for mouse cells responsive to antigenic stimulation by sheep erythrocytes. Proc Soc Exp Biol Med. 1965 Dec;120(3):868–873. doi: 10.3181/00379727-120-30678. [DOI] [PubMed] [Google Scholar]
- Lapp W. S., Möller G. Prolonged survival of H-2 incompatible skin allografts on F1 animals treated with parental lymphoid cells. Immunology. 1969 Sep;17(3):339–344. [PMC free article] [PubMed] [Google Scholar]
- Lawrence W., Jr, Simonsen M. The property of "strength" of histocompatibility antigens, and their ability to produce antigenic competition. Transplantation. 1967 Sep 5;5(5):1304–1322. doi: 10.1097/00007890-196709000-00009. [DOI] [PubMed] [Google Scholar]
- Möller G. 19S antibody production against soluble lipopolysaccharide antigens by individual lymphoid cells in vitro. Nature. 1965 Sep 11;207(5002):1166–1168. doi: 10.1038/2071166a0. [DOI] [PubMed] [Google Scholar]
- Möller G. Regulation of cellular antibody synthesis. Cellular 7S production and longevity of 7S antigen-sensitive cells in the absence of antibody feedback. J Exp Med. 1968 Feb 1;127(2):291–306. doi: 10.1084/jem.127.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Sjöberg O. Effect of antigenic competition on antigen-sensitive cells and on adoptively transferred immunocompetent cells. Cell Immunol. 1970 May;1(1):110–121. doi: 10.1016/0008-8749(70)90064-x. [DOI] [PubMed] [Google Scholar]
- Radovich J., Talmage D. W. Antigenic competition: cellular or humoral. Science. 1967 Oct 27;158(3800):512–514. doi: 10.1126/science.158.3800.512. [DOI] [PubMed] [Google Scholar]
- SNELL G. D., STEVENS L. C. Histocompatibility genes of mice. III. H-1 and H-4, two histocompatibility loci in the first linkage group. Immunology. 1961 Oct;4:366–379. [PMC free article] [PubMed] [Google Scholar]


