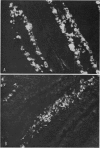
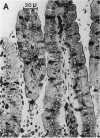
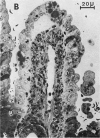
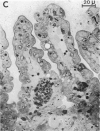
Abstract
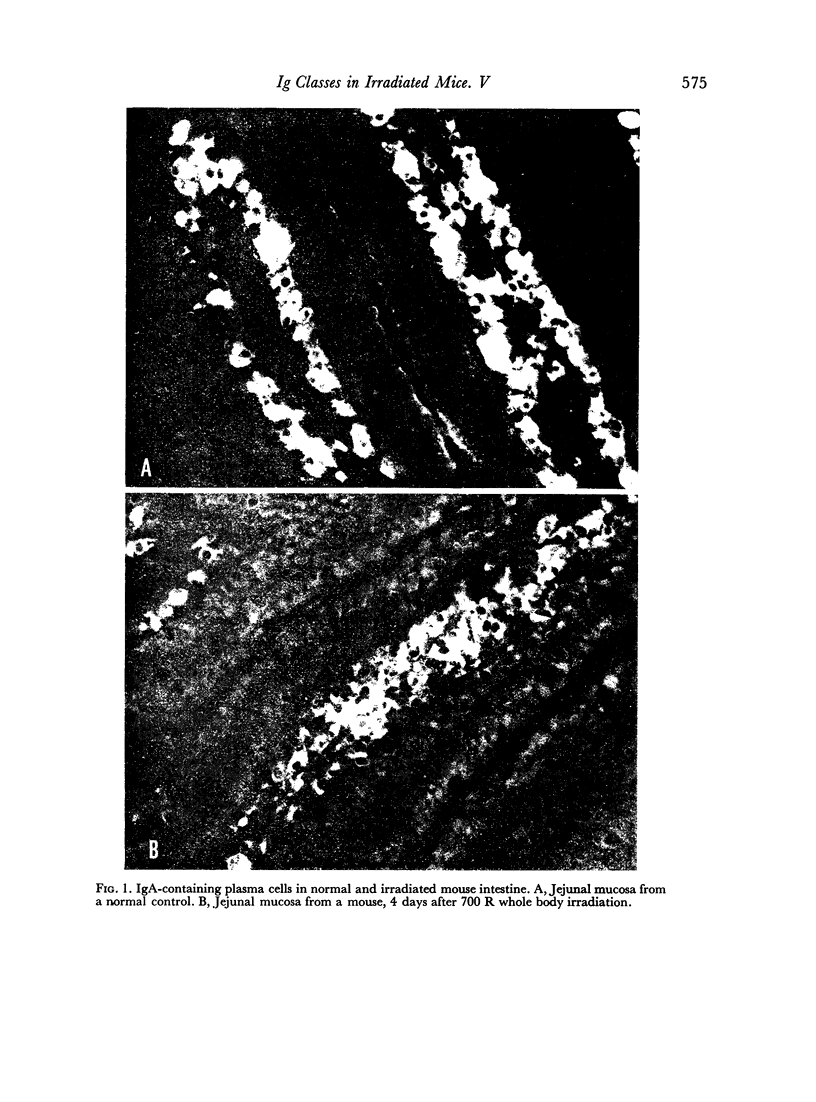
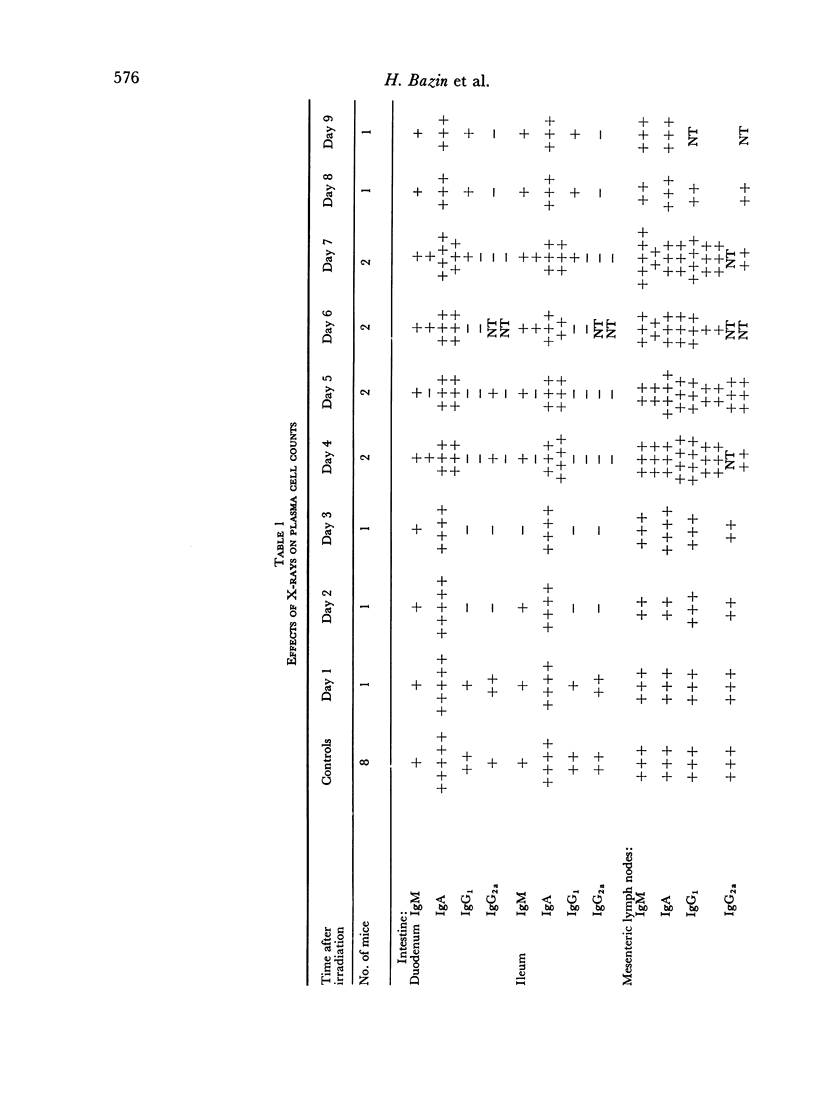
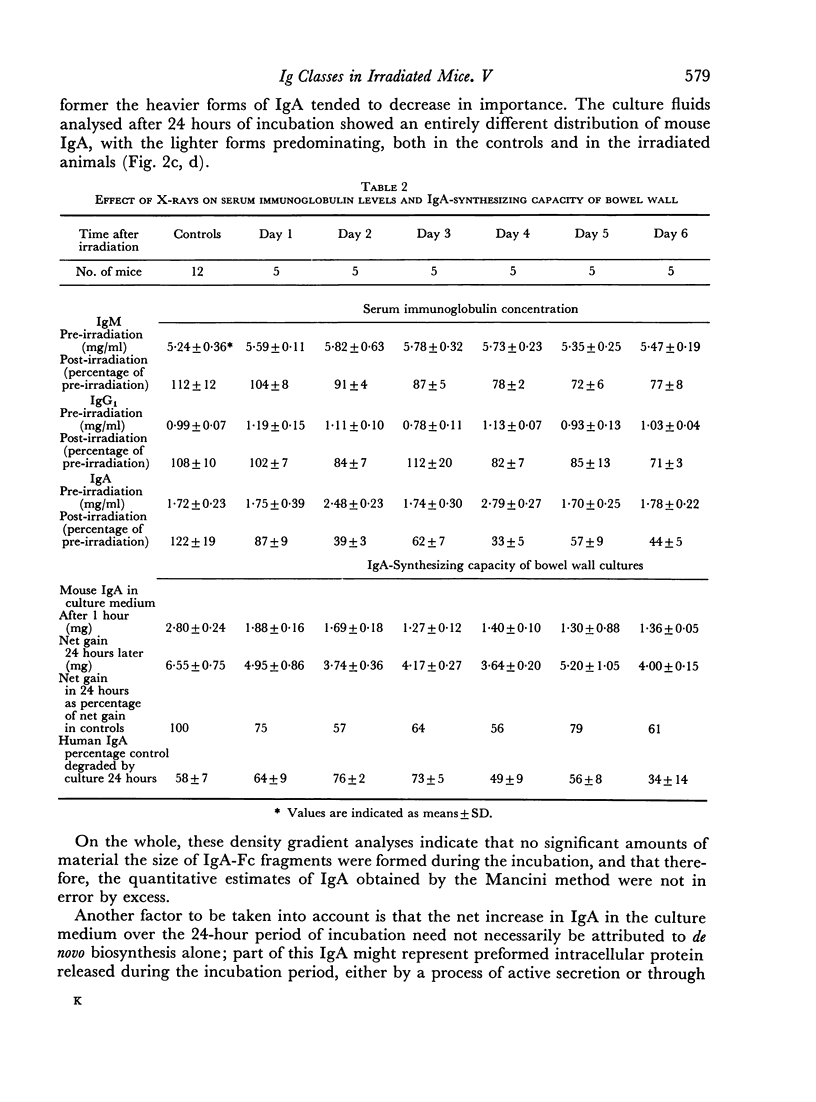
It was demonstrated in earlier publications that the acute and selective fall in serum IgA levels in irradiated mice was due to a specific failure to renew the plasma pool of this immunoglobulin. In the present work, it is shown that irradiation causes some reduction in the rate of biosynthesis of IgA, but that this phenomenon only partly accounts for the observed disturbance in the metabolism of IgA. This conclusion is based both on direct measurements on the rate of biosynthesis in vitro of IgA by the isolated small bowel wall of irradiated and normal C3H mice, and on immunohistological studies and plasma cell counts on the gut, spleen and lymph nodes of these animals.
On the other hand, it is shown that irradiated mice continue to excrete approximately normal amounts of IgA into their intestinal contents. The conclusion is that irradiation of the gut causes a rechannelling in the output of IgA synthesized by the plasma cells of the lamina propria, the major part being diverted to the intestinal lumen instead of being fed into the circulating pool of serum IgA.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., FAHEY J. L. Enzymatically produced subunits of proteins formed by plasma cells in mice. II. beta2a-Myeloma protein and Bence Jones protein. J Exp Med. 1962 Mar 1;115:641–653. doi: 10.1084/jem.115.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Doria G. The metabolism of different immunoglobulin classes in irradiated mice. 3. Effects of supra-lethal doses of x-rays. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(4):359–365. doi: 10.1080/09553007014550421. [DOI] [PubMed] [Google Scholar]
- Bazin H., Levi G., Heremans J. F. The metabolism of different immunoglobulin classes in irradiated mice. IV. Fate of circulating IgA of tumour or transfusion origin. Immunology. 1971 Apr;20(4):563–570. [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Maldague P., Heremans J. F. The metabolism of different immunoglobulin classes in irradiated mice. II. Role of the gut. Immunology. 1970 Mar;18(3):361–368. [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Malet F. The metabolism of different immunoglobulin classes in irradiated mice. Immunology. 1969 Sep;17(3):345–365. [PMC free article] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen D. V., Heremans J. F. Antibodies of the IgA type in intestinal plasma cells of germfree mice after oral or parenteral immunization with ferritin. J Exp Med. 1969 Oct 1;130(4):723–744. doi: 10.1084/jem.130.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- DETRICK L. E., LATTA H., UPHAM H. C., McCANDLESS R. Electron-microscopic changes across irradiated rat intestinal villi. Radiat Res. 1963 Jul;19:447–461. [PubMed] [Google Scholar]
- HUGON J., MAISIN J. R., BORGERS M. MODIFICATIONS ULTRASTRUCTURALES PR'ECOCES DES CELLULES DES CRYPTES DUOD'ENALES DE LA SOURIS APR'ES IRRADIATION PAR RAYONS X. C R Seances Soc Biol Fil. 1963;157:2109–2111. [PubMed] [Google Scholar]
- Hugon J., Maisin J. R., Borgers M. Delayed ultrastructural changes in duodenal crypts of x-irradiated mice. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;10(2):113–122. doi: 10.1080/09553006614550171. [DOI] [PubMed] [Google Scholar]
- KENNEDY J. C., TILL J. E., SIMINOVITCH L., MCCULLOCH E. A. RADIOSENSITIVITY OF THE IMMUNE RESPONSE TO SHEEP RED CELLS IN THE MOUSE, AS MEASURED BY THE HEMOLYTIC PLAQUE METHOD. J Immunol. 1965 May;94:715–722. [PubMed] [Google Scholar]
- Makinodan T., Nettesheim P., Morita T., Chadwick C. J. Synthesis of antibody by spleen cells after exposure to kiloroentgen doses of ionizing radiation. J Cell Physiol. 1967 Jun;69(3):355–366. doi: 10.1002/jcp.1040690312. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- QUASTLER H., HAMPTON J. C. Effects of ionizing radiation on the fine structure and function of the intestinal epithelium of the mouse. I. Villus epithelium. Radiat Res. 1962 Dec;17:914–931. [PubMed] [Google Scholar]
- Reuther A. M., Sassen A., Kennes F. Incorporation of labeled amino acids into serum and tissue proteins from normal and x-irradiated mice. Radiat Res. 1967 Mar;30(3):445–454. [PubMed] [Google Scholar]
- SARKAR N. K., DEVI A., HEMPELMANN L. H. Effects of irradiation on the incorporation of amino-acid into normal and regenerating rat liver. Nature. 1961 Oct 14;192:179–180. doi: 10.1038/192179a0. [DOI] [PubMed] [Google Scholar]
- Sado T. Functional and ultrastructural studies of antibody-producing cells exposed to 10,000 R in millipore diffusion chambers. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Feb 20;15(1):1–22. doi: 10.1080/09553006914550101. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F. Origin and molecular size of immunoglobulin-A in the mesenteric lymph of the dog. Immunology. 1970 Jan;18(1):27–38. [PMC free article] [PubMed] [Google Scholar]
- WEBBER B., GRAIG B. R., FRIEDMAN N. B. Cellular dynamics in the intestinal mucosa; quantitative measurements of the effects of nitrogen mustard and irradiation on cellular division and differentiation. Cancer. 1951 Nov;4(6):1250–1258. doi: 10.1002/1097-0142(195111)4:6<1250::aid-cncr2820040611>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]