Abstract
The purpose of the present experiments was to determine whether populations of lymphocytes from mouse or rat thoracic duct lymph deprived of the larger, dividing cells (large and medium lymphocytes) could still transfer adequate primary or secondary adoptive immune responses using syngeneic irradiated hosts. The transfer system used involved the antigen polymerized flagellin from Salmonella adelaide and assay of anti-H antibody levels in the recipients. Two methods of preparation of `small lymphocytes' were used, namely the glass bead column filtration method of Shortman and the agitated culture method of Gowans and Uhr (1966). Both of these yield lymphocyte fractions essentially free of dividing cells.
Unfractionated thoracic duct lymphocytes gave satisfactory adoptive immune responses in both species, primed cells being more effective than normal cells.
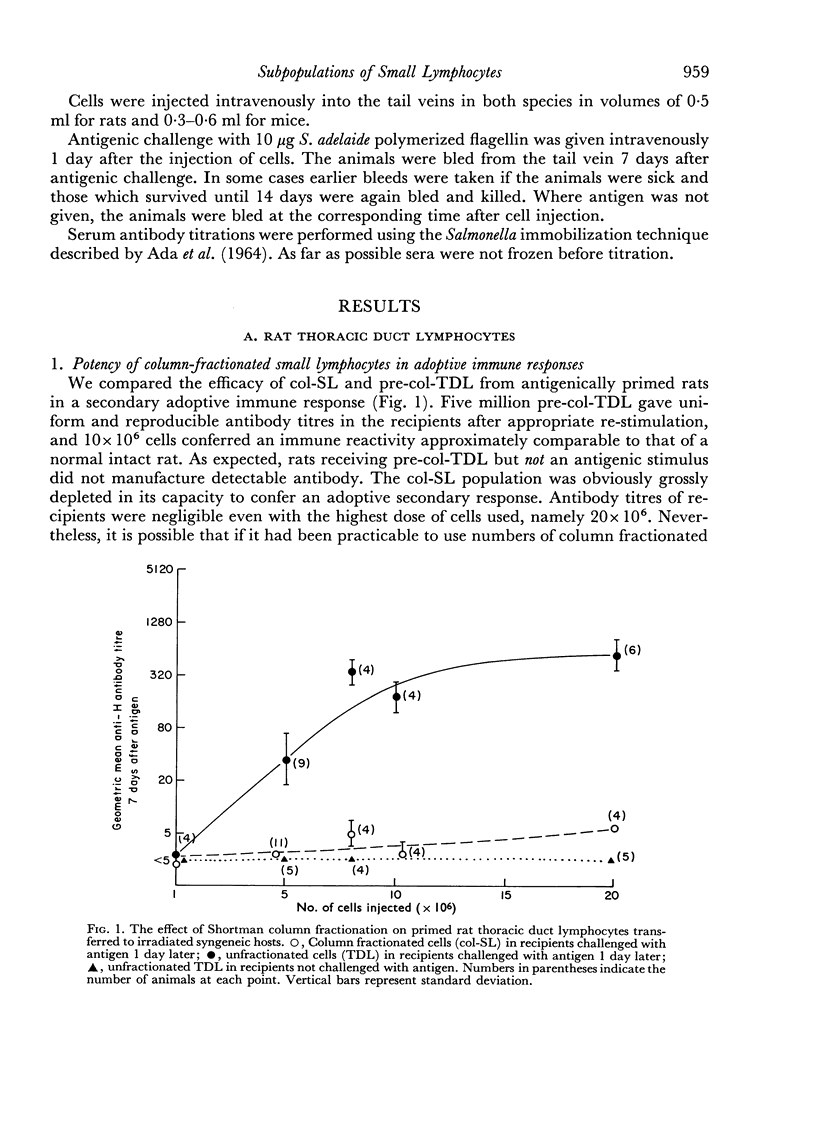
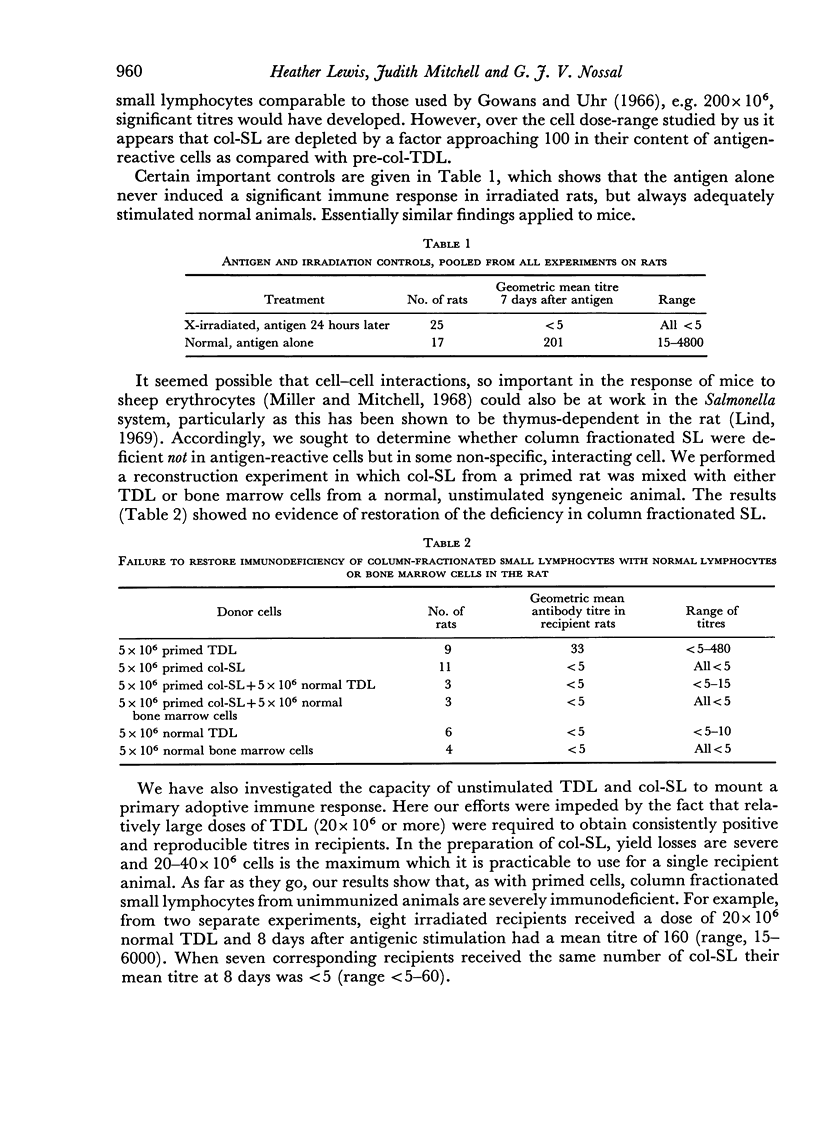
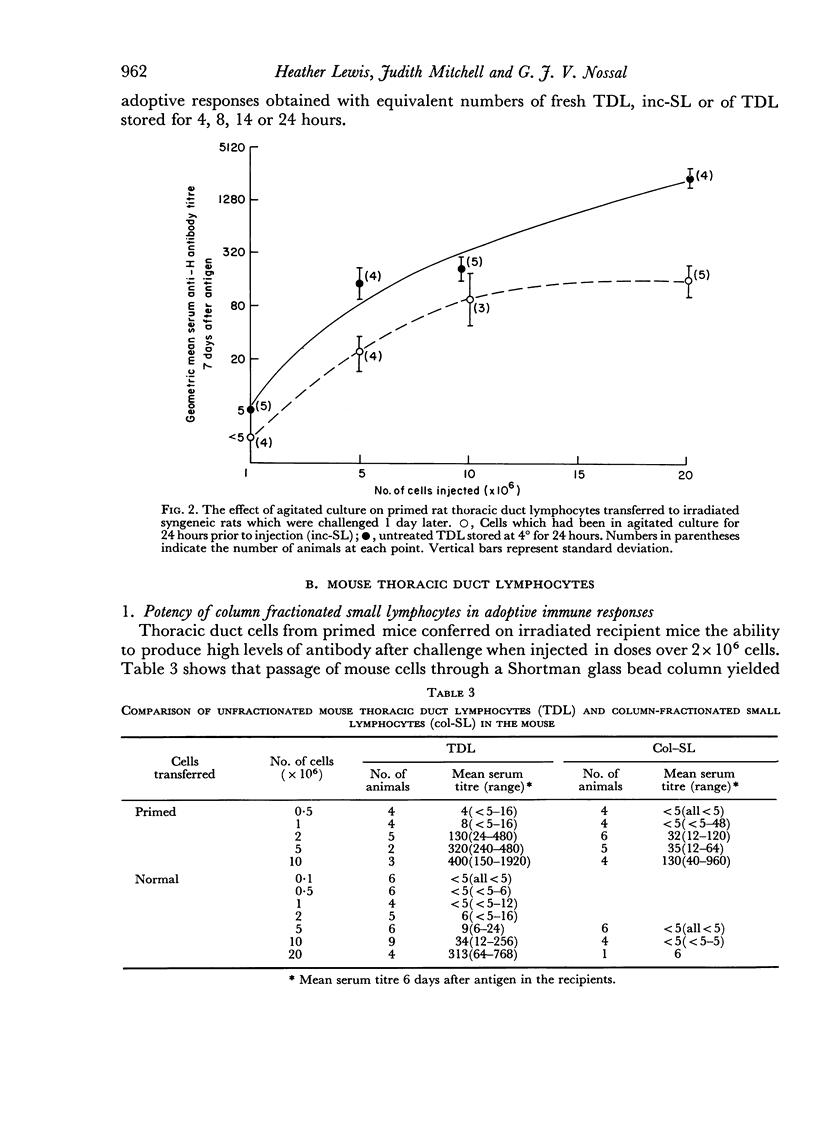
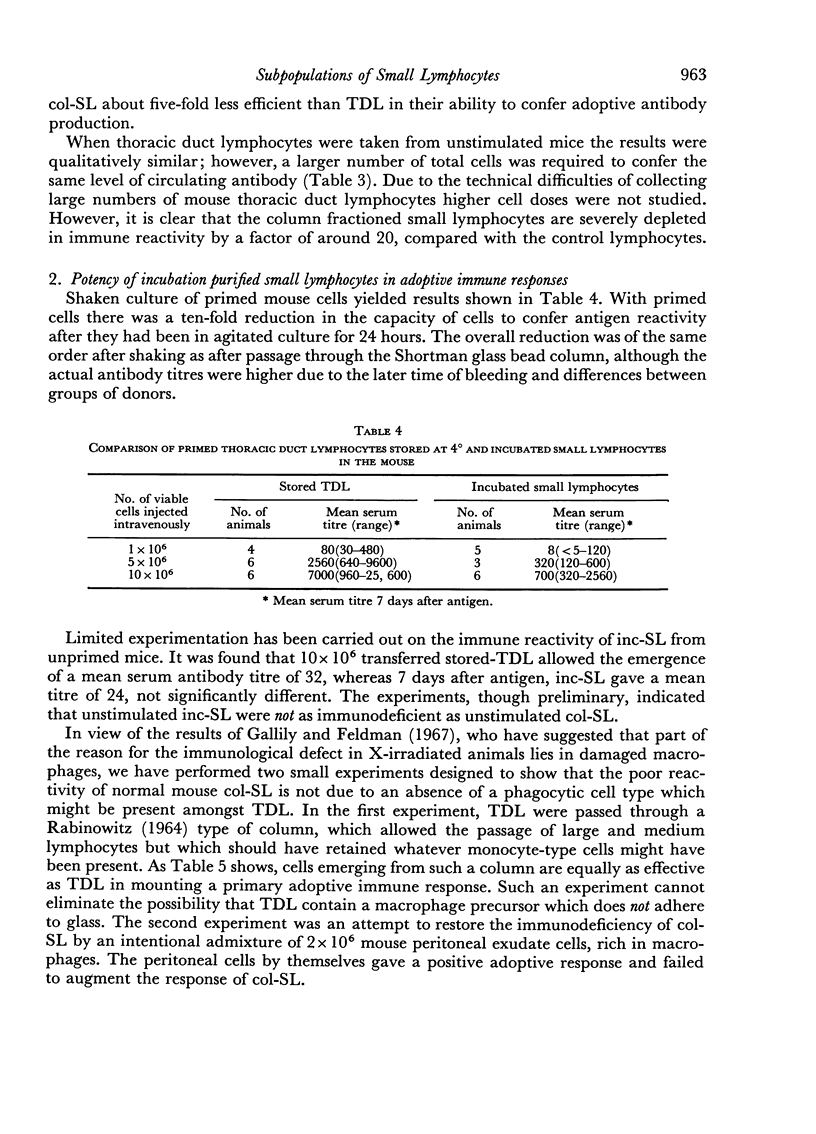
In both species and for both primary and secondary immune responses, the Shortman-column fractionated small lymphocytes (col-SL) were grossly impaired in their capacity to transfer adoptive responses. In contrast, the Gowans-type post-incubation small lymphocytes (inc-SL) gave rather better adoptive immune responses. Gowans and Uhr (1966) had found some enhancement of reactivity of inc-SL in comparison with normal lymph cells in another transfer system. We, however, found a mild impairment with unstimulated mouse and rat inc-SL and primed rat inc-SL and a slightly more marked impairment with primed mouse inc-SL.
We have confirmed the finding of Gowans and Uhr (1966) that lymphocyte populations free of dividing cells can mount an adoptive antibody response, showing that a proportion, at least, of the antigen-reactive cells can be described as `small lymphocytes'. The study also revealed the functional heterogeneity of morphologically similar lymphocytes, in that col-SL, morphologically the same as inc-SL, are functionally quite different; they appear to contain very few antigen-reactive cells. In the Salmonella system, therefore, the antigen-reactive thoracic duct cells can be described as lymphocytes which are fairly resistant to agitated culture but which fail to pass through Shortman columns.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. ANTIGENS IN IMMUNITY. I. PREPARATION AND PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA ADELAIDE. Aust J Exp Biol Med Sci. 1964 Jun;42:267–282. [PubMed] [Google Scholar]
- BOAK J. L., WOODRUFF M. F. A MODIFIED TECHNIQUE FOR COLLECTING MOUSE THORACIC DUCT LYMPH. Nature. 1965 Jan 23;205:396–397. doi: 10.1038/205396a0. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The fate of parental strain small lymphocytes in F1 hybrid rats. Ann N Y Acad Sci. 1962 Oct 24;99:432–455. doi: 10.1111/j.1749-6632.1962.tb45326.x. [DOI] [PubMed] [Google Scholar]
- Gallily R., Feldman M. The role of macrophages in the induction of antibody in x-irradiated animals. Immunology. 1967 Feb;12(2):197–206. [PMC free article] [PubMed] [Google Scholar]
- Gowans J. L., Uhr J. W. The carriage of immunological memory by small lymphocytes in the rat. J Exp Med. 1966 Nov 1;124(5):1017–1030. doi: 10.1084/jem.124.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill J. S., Legge D. G., Shortman K. Density distribution analysis of cells forming 19S hemolytic antibody in the rat. J Immunol. 1969 Mar;102(3):703–712. [PubMed] [Google Scholar]
- MITCHELL J. AUTORADIOGRAPHIC STUDIES OF NUCLEIC ACID AND PROTEIN METABOLISM IN LYMPHOID CELLS. I. DIFFERENCES AMONGST MEMBERS OF THE PLASMA CELL SEQUENCE. Aust J Exp Biol Med Sci. 1964 Jun;42:347–362. doi: 10.1038/icb.1964.34. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSSAL G. J., MAKELA O. Autoradiographic studies on the immune response.I. The kinetics of plasma cell proliferation. J Exp Med. 1962 Jan 1;115:209–230. doi: 10.1084/jem.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Cunningham A., Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968 Oct 1;128(4):839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. A., Evans W. H. Separation of bone marrow cells by sedimentation at unit gravity. Nature. 1967 May 20;214(5090):824–825. doi: 10.1038/214824a0. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ Y. SEPARATION OF LYMPHOCYTES, POLYMORPHONUCLEAR LEUKOCYTES AND MONOCYTES ON GLASS COLUMNS, INCLUDING TISSUE CULTURE OBSERVATIONS. Blood. 1964 Jun;23:811–828. [PubMed] [Google Scholar]
- Ruhenstroth-Bauer G., Lücke-Huhle C. Two populations of small lymphocytes. J Cell Biol. 1968 Apr;37(1):196–199. doi: 10.1083/jcb.37.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Szenberg A. The size and density distribution of fowl blood lymphocytes initiating a graft-versus-host reaction. Aust J Exp Biol Med Sci. 1969 Feb;47(1):1–9. doi: 10.1038/icb.1969.1. [DOI] [PubMed] [Google Scholar]
- Shortman K. The separation of different cell classes from lymphoid organs. 1. The use of glass bead columns to separate small lymphocytes, remove damaged cells and fractionate cell suspensions. Aust J Exp Biol Med Sci. 1966 Jun;44(3):271–286. [PubMed] [Google Scholar]
- Shortman K. The separation of different cell classes from lymphoid organs. II. The purification and analysis of lymphocyte populations by equilibrium density gradient centrifugation. Aust J Exp Biol Med Sci. 1968 Aug;46(4):375–396. doi: 10.1038/icb.1968.32. [DOI] [PubMed] [Google Scholar]
- Williams G. M. Antigen localization in lymphopenic states. I. Localization pattern following chronic thoracic duct drainage. Immunology. 1966 Nov;11(5):467–474. [PMC free article] [PubMed] [Google Scholar]


