Abstract
In humans, during standing the calf muscles soleus and gastrocnemius actively prevent forward toppling about the ankles. It has been generally assumed that these postural muscles behave like springs with dynamic stiffness reflecting their mechanical properties, reflex gain including higher derivatives, and central control. Here, for the first time, we have used an ultrasound scanner and automated image analysis to record the tiny muscular movements occurring in normal standing. This new, non-invasive technique resolves changes in muscle length as small as 10 μm without disturbing the standing process. This technical achievement has allowed us to test the long-established mechano-reflex, muscle spring hypothesis that muscle length changes in a spring-like way during sway of the body. Our results contradict that hypothesis. Muscle length changes in a non-spring-like manner: on average, shortening during forward sway and lengthening during backwards sway (paradoxical movements). This counter-intuitive result is a consequence of the fact that calf muscles generate tension through a series elastic component (SEC, Achilles tendon and foot) which limits maximal ankle stiffness to 92 ± 20% of that required to balance the body. Paradoxical movements cannot be generated by stretch reflexes with constant intrafusal drive but might be produced by reflex coupling of extrafusal (α) and intrafusal (β, γ) drive or by positive force feedback. Standing requires the predictive ability to produce the observed muscle movements preceded (110 ± 50 ms) by corresponding changes in integrated EMG signal. We suggest higher level anticipatory control is more plausible.
At its lowest level, postural control is widely thought to depend on mechano-reflex, feedback mechanisms that operate through the spinal cord and brainstem (Magnus, 1925; Shadmehr & Arbib, 1992; Mussa-Ivaldi & Bizzi, 2000; Massion et al. 2004). Specifically this belief is still held regarding the control of human standing (Gurfinkel et al. 1974, 1995; Fitzpatrick et al. 1992a, 1994, 1996; Horak & MacPherson, 1996; Winter et al. 1998; Schieppati & Nardone, 1999; Fitzpatrick, 2003; Masani et al. 2003). This reflex system generates forces in reaction to body movement. Forces are generated mechanically by the spring-like stiffness of the active muscles (Gurfinkel et al. 1974; Nashner, 1976; Horak & MacPherson, 1996; Winter et al. 1998). If the mechanical stiffness is inadequate on its own, the necessary additional force is generated by neural reflexes (Nashner, 1976; Houk & Rymer, 1981; Fitzpatrick et al. 1992a, 1994, 1996; Schieppati & Nardone, 1999; Fitzpatrick, 2003). The most basic of these is the (myotatic) stretch reflex where imposed lengthening of the muscle and muscle spindles causes increased activation and tension in the muscle. This is a negative feedback position controller; the muscles in effect act as springs with a dynamic stiffness set by the reflex gain. Delays between stretch and reaction can be minimized by phase advances within the nervous system (Neilson & Neilson, 1978; Fitzpatrick, 2003; Masani et al. 2003) and by a high reflex gain (Wolpert & Ghahramani, 2000).
In the literature on human standing it has been widely assumed, on purely anatomical grounds, that forward body sway is associated with a stretch of the calf muscles leading to the observed increase in activity and ankle torque (Gurfinkel et al. 1974; Fitzpatrick et al. 1992a,b, 1994, 1996; Horak & MacPherson, 1996; Winter et al. 1998, 2001; Schieppati & Nardone, 1999; Gatev et al. 1999; Masani et al. 2003). While this seems plausible, the changes in length of the postural muscles have never been directly observed under postural conditions. In particular one must distinguish between the muscle as the contractile element and the muscle as the muscle–tendon complex (Fig. 1). In this paper we refer to muscle as the contractile element. If we define the muscle spring theory as the idea that the length of the contractile element signals joint angle, and that resistance to muscle elongation is enhanced by stretch reflexes, then a central assumption is that forward sway of the body is associated with a stretch of the calf muscles. It seems imperative that this muscle spring theory should be tested by direct observation. Is the muscle stretched during forward sway or is it not? If the contractile element is stretched and lengthened during forward sway this indicates that the muscle is acting in a spring-like manner supplemented by the stretch reflex mechanism. Unorthodox, independent changes in muscle length that do not reflect position of the body are incompatible with the simple muscle spring theory above and would suggest a more complex peripheral feedback process or even a higher level control process (Loram & Lakie, 2002a; Lakie et al. 2003).
Figure 1. Postural muscles of standing and the dynamic bias model.
A, the calf muscles soleus and gastrocnemius connect the Achilles tendon to the back of the lower leg bones and the back of the knee, respectively. B, sonograph of the gastrocnemius medialis and soleus muscles taken from the medial aspect. a, proximal aponeurosis of gastrocnemius medialis; b, distal aponeurosis of gastrocnemius; c, distal aponeurosis of soleus; d, proximal aponeurosis of soleus. b and c are morphologically distinct but moved as a unit and were tracked using a single set of markers. The size of the image is 4.5 cm × 4.5 cm. C, the dynamic bias model. The body is represented by an inverted pendulum with its centre of mass (CoM) indicated. The gastrocnemius and soleus muscles together are represented by the contractile element (CE). These muscles act through a spring-like element which connects them to the ground through the foot. The total stiffness of this elastic link is represented by K. The system operates by dynamically altering the length of the CE thus altering the position of one end of K. We refer to the length of the CE as the bias of the spring. In angular terms, the length of the spring is given by the angle of the CoM relative to the vertical (θ) minus the length of the bias (θ0). Ankle torque is then given by T=K(θ−θ0).
Technically, this muscle spring theory is difficult to test, particularly under postural conditions. The muscle movements are predicted to be very small (120 μm) (Lakie et al. 2003). Very recently, we tracked the much larger muscle movements present during exaggerated, voluntary sways and we confirmed that paradoxical muscle movements are the norm (Loram et al. 2004). Here, using automated tracking of ultrasound images, we observe for the first time the tiny, involuntary movements of the muscles that occur during the postural sway of normal standing. The imaging technique is entirely non-invasive and does not disturb the standing process.
In previous experiments we have tested the consequences of a compliant tendon linkage on the muscular control of a real, human-proportioned inverted pendulum (Lakie et al. 2003). Subjects stabilized the pendulum by manually pulling on a weak spring which was attached to the pendulum. Using this dynamic bias model we predicted that, on average, the calf muscles shorten as the body sways forwards and lengthen as the body sways to the vertical (paradoxical muscle movements). In this paper, we test the validity of this prediction under postural conditions. Namely, paradoxical muscle movements are the norm in quiet standing and that this is a consequence of the compliant series elastic linkage of the calf muscle.
Methods
Procedure and measurements
Ten healthy subjects, aged between 25 and 49 years, stood quietly, with neither foot in front of the other and feet at a normal distance apart. Subjects were asked to stand for six trials of 40 s in which three trials with eyes open were alternated with three trials with eyes closed. The subjects gave informed consent, and the study was approved by the local human ethics committee and conformed to the principles of the Declaration of Helsinki.
Combined ankle torque from both legs was measured using a purpose-built foot-plate with a single axis of rotation orientated with the ankle and a vertically mounted load cell. Surface EMG activity (Neurolog) was recorded from the left soleus and gastrocnemius medialis, amplified (10 000 ×) and band-pass filtered at 60–500 Hz. All signals were sampled at 1000 Hz and recorded to 16-bit resolution on computer (Measurement computing PCI-DAS6036, MATLAB). The EMG signals were digitally rectified and integrated. All signals were subsequently down-sampled to 100 Hz. The position of the body centre of mass (CoM) was calculated by filtering the combined torque signal (Loram & Lakie, 2002b). We also measured ankle angle using a laser range finder (YT25MGV80, Wenglor Sensoric, Germany) that was mounted on the support surface and reflected off the left shin. Comparison of ankle angle with CoM angle enables us see when body sway was not identified with ankle angle. An ultrasound probe (Esaote Biomedica AU5 scanner, 7.5 MHz linear-array probe) was fixed along the calf to provide a parasagittal-plane view of the underlying muscles (Fig. 1). Images from the ultrasound scanner were digitized at 25 frames s−1 using a frame grabber (Data Translation DT3120) and synchronously recorded on computer using MATLAB software.
The method for tracking and calculating changes in muscle length relative to a single base frame has already been reported (Loram et al. 2004). Markers are placed on the proximal and distal aponeuroses of the gastrocnemius and soleus muscles (Fig. 1B). When either muscle shortens, the distal and proximal aponeuroses move approximately antiparallel relative to each other, and the angle of the fibres become more obtuse relative to the aponeurosis. By tracking and calculating the relative movement between both proximal and distal aponeuroses, any relative motion between the scanner probe and the muscle was eliminated and an estimate was formed of changes in muscle length. In this calculation, the muscles are regarded as elements that shorten along the direction of the muscle tendon complex (Fig. 1C).
In this paper we are examining changes in muscle length which are an order of magnitude smaller than those reported in our earlier study which examined large, voluntary sways of the body. The larger muscle movements reported previously and the larger muscle movements present during quiet standing can be verified visually by inspecting the motion of the markers on the movie sequence of ultrasound images. For illustration we have provided a movie sequence showing visible changes in muscle length during quiet standing (see Supplementary Material). However, many of the changes in muscle length are difficult to discern by eye. This new technique underpins the claims that we make. So what confidence can we place in the measurements obtained?
For each muscle, the change in muscle length was calculated eight times by eight independent pairs of markers on the proximal and distal aponeurosis. The tracking procedure calculates the movement of a square 15 pixels × 15 pixels centred on the marker. These squares were positioned so as not to overlap. The tracking procedure uses 2-dimensional cross-correlation to identify the change in position of the square between two frames. As the muscle shortens the image changes and thus a perfect correlation between two squares is never attained. The change in image is less with small contractions than with large contractions and thus, the absolute error is less for smaller contractions. Based on eight marker pairs, for each frame, the mean 95% confidence intervals for changes in muscle length relative to a common base frame were ± 130 μm and ± 40 μm for soleus and gastrocnemius, respectively, which is less than that reported for the larger voluntary sways. We regarded these values as the accuracy of the measurements over the entire trial duration. The error is greater for the deeper soleus muscle because the ultrasound scanner is able to resolve less detail at the depth of the proximal soleus aponeurosis.
By expressing changes in muscle length relative to a single, common base frame we obtain a consistent reference for changes in muscle length for an entire trial. However, what is the smallest change in muscle length that can be reliably observed between consecutive frames? Using the eight marker pairs, the changes in muscle length between consecutive frames were tracked with a mean 95% confidence interval of 14 μm and 9 μm for soleus and gastrocnemius, respectively. We regard these values as the resolution of the technique. The difference between the resolution and trial duration accuracy can be explained by the tendency of the image content within the squares to alter as well as change position through the duration of the trial. The result is analogous to the DC drift introduced by an electronic amplifier.
The changes in muscle length are actually calculated as pixels on the image and are converted to distance by the scale provided on the image by the manufacturer. Thus the accuracy of all length changes is limited to the accuracy of the scanner scale which is ± 4.4% according to the manufacturer.
In these experiments we are measuring changes in muscle length along the line of the muscle–tendon complex and we assume that the proximal and distal aponeuroses move relative to each other as non-deformable objects. This is thought to be a good assumption (Magnusson et al. 2003). However, these sonographs show approximately 4.5 cm of muscle whereas the entire length of the muscle belly is 32 cm for soleus and 22 cm for gastrocnemius. Thus in these measurements, we cannot account for the error introduced by non-uniform shortening along the length of the muscle.
Finally, due to the angle of pennation, the changes in muscle length that we measure are not the same as the changes in fascicle length. To measure changes in fascicle length it is necessary to observe both ends of a complete muscle fascicle (Herbert et al. 2002).
As they are so small, one would like some independent verification of the changes in muscle length. The EMG measurements provide an independent measurement that can be used to verify the pattern if not the size of the changes in muscle length. When the tendon is compliant we expect increases in integrated EMG activity to be associated with shortening of the muscle. Additionally, the dynamic bias model (Fig. 1) can be used to predict changes in muscle length from changes in CoM angle and a value for SEC stiffness that is broadly consistent with previously measured values (Loram & Lakie, 2002b). Even if the stiffness chosen is not quite accurate, the predicted pattern if not the exact size of changes in muscle length should agree. In these experiments, using the dynamic bias model amounts to testing the changes in muscle length using the torque signal. This method is limited by the necessary assumption for the present experiments that both legs participate synchronously and in a 1: 1 ratio of ankle torque. This is unlikely to be true in particular cases, although it should be true on average.
The ultrasound scanner and frame grabber introduced a time delay between movements of the muscle and the digital images recorded on the computer. This time delay was measured by simultaneously recording the position of a wire oscillating in a beaker of water and tracking the wire using the ultrasound scanner. The time delay of 80 ± 40 ms was subtracted from the acquisition time of each ultrasound frame.
The dynamic bias model
We predicted the correlation between muscle length and the CoM angle using a dynamic bias model (Fig. 1C). The dynamics of the body is represented by a single mass at the CoM (Winter et al. 1998; Gatev et al. 1999), where I is the moment of inertia, θ is the angle of the CoM, m is the mass of the body above the ankles, g is the acceleration due to gravity, h is the height of the CoM above the ankles and T is the ankle torque applied to the CoM through both legs:
| (1) |
This equation holds for as long as the subject is maintaining balance by the ankle strategy.
Ankle torque generated by the calf muscles is transmitted through a linear spring (Achilles tendon in series with the flexible foot) where K is the spring stiffness and θ0 is the offset or bias of the spring. This bias is set by the length of the contractile element which represents the active belly of the muscles:
| (2) |
The spring is conveniently expressed as a fraction, c, relative to the toppling torque per unit angle (load stiffness), mgh, of the CoM:
| (3) |
Eqns (1), (2) and (3) give the angular changes in bias:
| (4) |
The fraction I/mgh scales with body height and was calculated for each subject using the height of the subject and a regression equation from previous measurements.
The normalized cross covariance, rxy, between bias (muscle length, x) and CoM angle (y) gives the correlation between the two zero referenced signals for a variety of time shifts τ and is calculated using:
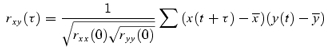 |
(5) |
A normalized cross covariance of +1 indicates that the two signals are identical, −1 indicates the two signals are a perfect negative reflection of each other and 0 indicates that there is no correlation between them.
This cross-correlation method provides a simple indicator of whether the prevailing muscle movements are orthodox or paradoxical and whether the relative stiffness c is greater or less than 100% (see Fig. 3C). It also provides an estimate of the relative stiffness operating at the prevailing size and frequency of sways in quiet standing. This is useful because it is known that the ankle stiffness varies considerably with the size of ankle movement (Kearney & Hunter, 1982; Evans et al. 1983). This normalized cross correlation method predicts the relative stiffness from the pattern of changes in muscle length and CoM angle and is insensitive to errors in the scaling of these quantities.
Figure 3. Correlation between muscle length and body sway.
All three panels show the normalized cross-correlation function between CoM angle and muscle length. The cross-correlation function shows the correlation between these two signals at different values of time shift between the two quantities. A positive time shift would indicate that the muscle length was advance of the CoM angle. A, soleus muscle length. B, gastrocnemius medialis muscle length. C, predicted correlations using the dynamic bias model. Predictions are for spring stiffness expressed relative to the toppling torque per unit angle of the pendulum (load stiffness). The values are 60, 80, 90, 100, 110, 120 and 160%.
The relationship between muscle length and CoM angle can also be examined in the frequency domain. The gain and phase components of the transfer function TθL were estimated empirically from the muscle length, CoM angle, cross power spectrum (PLθ) and the power spectrum (Pθθ) of the CoM angle (Control system toolbox, MATLAB, The Mathworks), by:
| (6) |
A coherent frequency relationship between muscle length and CoM angle can be predicted from the time domain equation of the dynamic bias model. Laplace transformation of eqn (4) gives the transfer function:
| (7) |
For sustained oscillations, s= i2πf (Schwarzenbach & Gill, 1992) where i is the square root of minus one, and so the transfer function between linear changes in muscle length (L) and CoM angle (θ) can be expressed as:
| (8) |
According to the dynamic bias model, the frequency relationship between muscle length and CoM angle is determined biomechanically by the parameters given in eqn (8). Muscle length is also controlled by the subject in response to and in anticipation of changes in CoM angle. However, what the subject does by modulating muscle length, is determine the frequency range in which the peak power of CoM oscillations occur. Subject modulation of muscle length cannot alter the biomechanical relationship between muscle length and pendulum motion.
We can check whether the form of the empirical transfer function is consistent with the dynamic bias model and we can estimate the value of the SEC stiffness from the empirical transfer function. This estimate is not a direct measurement. It rests on the assumption that eqn (8) is a correct description of the relationship between muscle length and CoM angle and thus it should be regarded as a prediction to be compared with values obtained by a direct measurement process (Kearney & Hunter, 1982; Loram & Lakie, 2002b; Casadio et al. 2005).
By rearranging eqn (8), the complex stiffness of the SEC was estimated as a function of frequency from the empirical transfer function:
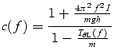 |
(9) |
Non-inverted pendulum-like movements are more likely to occur at higher frequencies due to the decreased moment of inertia about joints other than the ankle. Also the signal-to-noise ratio of the measurements decreases with frequency. Thus the reliability of these calculations is likely to decrease as frequency increases.
We tested the reliability of this method of calculating the empirical transfer function and estimating the complex SEC stiffness as follows. In our previously published experiment (Lakie et al. 2003) subjects used a variety of series springs of known stiffness to manually stabilize a real inverted pendulum while we recorded the changes in bias of the spring and angle of the pendulum. Using these data we calculated the empirical transfer function and calculated the variation of stiffness with frequency as described above, and compared the calculated relative spring stiffness, c, with the previously measured relative spring stiffness. The frequency domain calculations estimated the constant spring stiffness correctly with a mean difference error of 11% for all springs at all frequencies in the range 0–3 Hz.
Results
Muscle movements in quiet standing
For the illustrative subject standing with eyes open (Fig. 2) there is clear evidence of very small, active changes in muscle length. The sense of these changes is consistently paradoxical. That is, muscle shortening as the body sways forwards and muscle lengthening as the body sways backwards to the vertical. In this record, there is slow drift of the body towards the vertical of about 0.6 deg over 35 s (Fig. 2B) and during this time the length of the soleus and gastrocnemius muscles increases by approximately 1 mm (Fig. 2C). Smaller, short duration sways are also accompanied by clear contrary changes in muscle length. This result contradicts naive expectation based on the anatomical arrangement of the calf muscles. The soleus and gastrocnemius muscles follow a similar pattern but do not shorten identically, possibly because gastrocnemius is a two-joint muscle whereas soleus only crosses the ankle joint.
Figure 2. Example of paradoxical muscle movements.
Records are shown of a subject standing quietly and symmetrically with eyes open. A, combined ankle torque from both legs. B, left ankle angle (continuous line) and estimated CoM angle (dashed line) C, muscle length, gastrocnemius medialis (continuous line) and soleus (dashed line). D, predicted changes in muscle length using the dynamic bias model with moment arm of 5 cm and best fit stiffness of 48%. E, integrated EMG activity for gastrocnemius medialis F, integrated EMG activity for soleus. For both EMG records, τ= 200 ms. Muscle lengths are shown relative to typical mean muscle belly lengths of 320 and 220 mm, respectively, for soleus and gastrocnemius.
The tiny changes in muscle length are beautifully related to changes in EMG activity such that increases in EMG activity are associated with a shortening of the muscle (Fig. 2C, E and F). It may be surprising to see the significance of tiny fluctuations in EMG activity which might ordinarily be dismissed as noise. As these muscle movements have never been observed before, the close correspondence between EMG and changes in muscle length gives confidence in the high sensitivity of the muscle length tracking procedure.
The size and pattern of changes in muscle length have been predicted (Fig. 2D) using the dynamic bias model (Fig. 1) with an average Achilles tendon moment arm length of 5 cm (Maganaris et al. 1998) and a best-fit spring stiffness of 44%. The model relates changes in muscle length to changes in ankle torque and shows that as the muscle tension increases the muscle length shortens. In this example, the agreement between predicted and actual changes in muscle length is generally good and also gives credibility to the measured changes in muscle length. The agreement is less good at around 17 s and 32 s which corresponds with a disparity between the CoM angle and ankle angle. Note the EMG records indicate that the muscle tracking is correct and the model is incorrect. So at these times, the ankle angle is not a good predictor of CoM angle due to angular movement at other joints such as the knee joint.
Paradoxical muscle movements are the norm in human standing
For all 10 subjects, the soleus muscle length is on average correlated negatively with CoM angle (Fig. 3A). Four of the correlations are weak indicating little relationship between sway angle and muscle length. For eight of the subjects, gastrocnemius is also correlated negatively with CoM angle (Fig. 3B). Three of the negative correlations are again weak. For two subjects there is a weak positive correlation between CoM angle and gastrocnemius muscle length. There were no systematic differences in correlation between the eyes open and eyes closed conditions. For a spring-like change in muscle length, there would be a positive correlation between muscle length and CoM angle. The negative correlation implies a process which is not spring-like. A weak correlation implies that muscle length is changed independently of CoM position. According to the dynamic bias model predictions (Fig. 3C), the cross-correlations shown indicate a mean stiffness of 92 ± 20% relative to the toppling torque per unit angle of the CoM.
Using cross-correlation analysis, the time delay of maximum correlation between integrated EMG activity (for this calculation τ= 100 ms) and muscle length is 110 ± 50 ms with the changes in muscle length following changes in EMG activity (Fig. 4A and B). Allowing for the median integration delay of 50 ms, this means that α motor neurone activity is modulated approximately 160 ± 50 ms ahead of muscle length. This is not the latency between an EMG stimulus and the onset of contraction, it is the average time between changes in EMG and corresponding changes in muscle length.
Figure 4. Correlation between EMG activity and muscle length.
A and B, show the normalized cross-correlation function between integrated EMG activity (τ= 100 ms) and muscle length for soleus and gastrocnemius, respectively. The mean for all 10 subjects is shown by the thick continuous lines. The variation is shown by the dashed lines which indicate the mean of six trials for each subject. A positive time indicates that muscle length lags EMG activity. For soleus and gastrocnemius, respectively, C and D, show the peak correlation coefficient for muscle length lagging EMG activity plotted against the stiffness of the SEC The stiffness of the SEC was calculated using the cross correlation between muscle length and CoM angle as shown in Fig. 3, and is expressed relative to the load stiffness. Each dot represents the mean of six trials for one subject.
Figure 4 also shows that with the exception of gastrocnemius in one subject, changes in muscle length are correlated negatively with changes in EMG activity (Fig. 4A and B). This result is consistent with a compliant SEC and the cases with high correlation between EMG activity and muscle length provide independent validation that the pattern of muscle movements is genuine. One would expect an increased negative correlation between muscle length and EMG activity when the SEC is less stiff because the muscle length is actively modulated with greater amplitude (Lakie et al. 2003). This prediction is confirmed significantly for gastrocnemius (Pearson's correlation coefficient, N= 10, P= 0.043) and there are some notably high correlations between muscle length and EMG activity when the SEC stiffness is low. This prediction is not confirmed for soleus (N= 10, P= 0.23) although it is not impossible that the prediction would be confirmed with a greater range of SEC stiffness. The pattern of changes in muscle length for soleus is actually very similar to those of gastrocnemius. Inspection of Fig. 2E and F illustrates our general observation that soleus integrated EMG activity is modulated much less in relation to the tonic level of activation (17%s.d. mean−1) than gastrocnemius (38%s.d. mean−1). Because they are mechanically coupled, the shortening and lengthening of soleus could be enhanced by the contraction and relaxation of gastrocnemius and not solely controlled by changes in soleus activation.
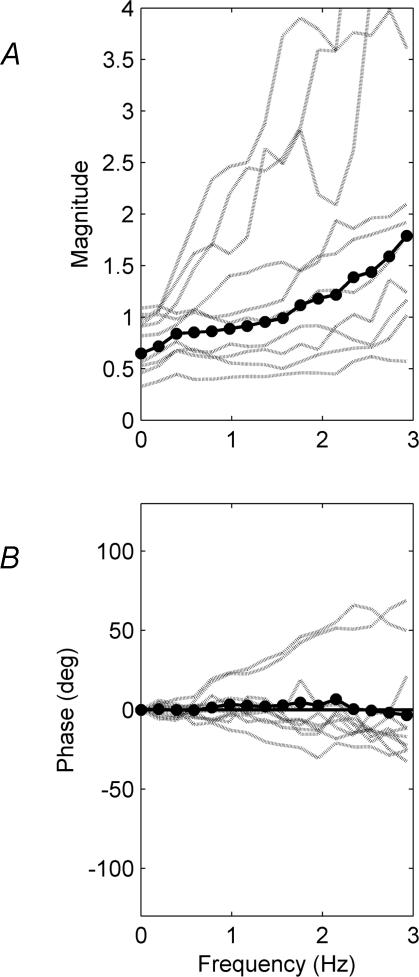
The relationship between changes in muscle length and changes in CoM angle can also be viewed in the frequency domain. On average, both muscles soleus (Fig. 5D) and gastrocnemius (Fig. 5E) are modulated to change length 180 deg out of phase with CoM across the frequency range 0–3 Hz. This is consistent with a spring-like SEC of relative stiffness less than 100% (Fig. 5F). This frequency range encompasses > 99.99% of the power of CoM oscillations and 99.8% of the power of changes in muscle length. For one subject there is evidence of orthodox muscle length changes in soleus at tonic levels of contraction (0 Hz) and for two subjects gastrocnemius muscle length changed in phase with CoM angle for up to 0.5 Hz. The gain (mm deg−1) between changes in muscle length and changes in CoM angle increases with frequency (Fig. 5A and B) in a manner that is consistent with a spring like SEC (Fig. 5C). This presentation of results agrees with the cross-correlation analysis that paradoxical muscle movements resulting from a compliant SEC are the norm in quiet standing.
Figure 5. Transfer function between muscle length and CoM angle.
A and B, show the absolute gain (mm deg−1) relating changes in muscle length to changes in CoM angle for frequencies up to 3 Hz. The gain and phase of the mean transfer function, averaged from all 10 subjects, is shown by the continuous lines (•–•). The variation is shown by the dashed lines which indicate the mean of six trials for each subject. C, shows the predicted transfer function for the dynamic bias model with moment arm of 50 mm and a variety of stiffness values of the SEC The continuous line is 1.0, the dash–dot lines are 0.4 and 0.7 and the dashed lines are 1.4 and 2.0 relative to the load stiffness of the inverted pendulum. D–F, show the corresponding phase relationships. A negative phase indicates that muscle length is behind CoM angle.
From close inspection of (Fig. 5A–C) it can be seen that the stiffness of the SEC increases with frequency. Calculation of the stiffness of the SEC at each frequency is presented in Fig. 6. It can be seen that on average the relative stiffness of the SEC rises from 65% at tonic contractions (0 Hz) to 110% at 2Hz and 180% at 3 Hz (Fig. 6A). The average phase of this stiffness is close to 0 deg for frequencies 0–3 Hz indicating that the SEC is spring-like with a minimal viscous component for these frequencies.
Figure 6. Dynamic stiffness of the SEC.
A and B, show the absolute gain and phase of the complex stiffness of the SEC In accordance with the dynamic bias muscle there is only one SEC and so both soleus and gastrocnemius muscles have been averaged together. The mean for all 10 subjects is shown by the continuous lines (•–•). The variation is shown by the dashed lines which indicate the mean of six trials for each subject. A non-zero phase indicates a viscous component to the complex stiffness.
Discussion
Confirmation of paradoxical muscle movements and the compliant SEC
Under postural conditions, the present results validate the prediction of our previous work (Lakie et al. 2003). Namely, paradoxical muscle movements are the norm in quiet standing and this is a consequence of the compliant series elastic linkage of the calf muscle. The active modulation of muscle length during quiet standing is a new observation revealed by this new technique using an ultrasound scanner to track the tiny involuntary muscle movements. The pattern of changes in muscle length is supported by corresponding fluctuations in EMG activity particularly by gastrocnemius EMG.
Paradoxical muscle movements (Fig. 3A and B) are exactly what is predicted (Fig. 3C) by a low stiffness of the SEC (Lakie et al. 2003) and the time domain estimate of 92 ± 20% relative to the load stiffness is consistent with previous measurements of intrinsic ankle stiffness during quiet standing (Loram & Lakie, 2002b). Using frequency analysis, the complex stiffness of the SEC is estimated to be almost entirely spring-like and not viscous in the frequency range relevant to quiet standing. The result that the stiffness increases with frequency should be interpreted with care. At higher frequencies, the amplitude of CoM oscillation decreases. The increase in stiffness may be due to the decrease in size of the ankle movements rather than the change in frequency. These results are in good agreement with the fact that ankle stiffness increases considerably as the size of ankle movement decreases (Kearney & Hunter, 1982; Evans et al. 1983). The low-frequency estimate of SEC stiffness of 65% is in good agreement with values measured for ankle movements of 1 deg (64 ± 8%, Casadio et al. 2005), which are movements comparable with the drift obtained over many seconds or tens of seconds. The estimate of SEC stiffness at a mean frequency of sway of 0.5 Hz is 85% which is in good agreement with direct measurements of the SEC prevailing during the smaller sways of quiet standing (91 ± 23%, Loram & Lakie, 2002b).
It has long been realized that tendon compliance needs to be considered in muscle models and that tendon compliance can have a considerable effect on how muscle length changes (Rack et al. 1983; Rack, 1985; Zajac, 1989; Zajac & Gordon, 1989). For example, the stiffness of the Achilles tendon and foot and their relevance to energy storage–release in locomotion has been known for some time (Alexander & Bennet-Clark, 1977; Ker et al. 1987; Fukunaga et al. 2001). However, the relevance of low tendon and foot stiffness to the control of standing is only now being recognized (Morasso et al. 1999; Morasso & Schieppati, 1999; Morasso & Sanguineti, 2002; Herbert et al. 2002; Loram & Lakie, 2002a,b; Lakie et al. 2003; Loram et al. 2004). A low-stiffness system requires additional active torque modulation (Loram & Lakie, 2002a) associated with bias adjustments which are generally paradoxical or uncorrelated with CoM angle (Lakie et al. 2003) in order to counteract the instability of the muscle–tendon-load system.
The role of intrinsic ankle stiffness in standing
Our results demonstrate the fallacy of the long-established idea that intrinsic ankle stiffness is sufficient to stabilize quiet standing (Gurfinkel et al. 1974; Nashner, 1976; Horak & MacPherson, 1996; Winter et al. 1998). On the contrary, the stiffness is dominated by the weakest link in the series chain, and that weakest link, the SEC is insufficient for static stabilization. If the activation and length of the muscle remained constant, the person would continuously fall forwards because the tendon spring would not be stiff enough to restrain them. However, with a relative stiffness of ∼90% due to tonic contraction of the calf muscles, the SEC partially compensates for the effect of gravity. The toppling torque per unit angle is effectively reduced to 10% of its full, uncompensated value. This presents a less unstable load for the nervous system to control because the load will accelerate less for a given fluctuation in torque produced by the calf muscles.
The role of the muscle spring in standing
Our new observations provide clear evidence against the simple muscle spring theory where length of the contractile element signals joint angle. It has been assumed that as the body slowly sways forwards, the calf muscles are stretched, the length of the muscle signals movement of the body and that this signal stimulates a spring-like or viscous-like increase in muscle activation that aids stability (Shadmehr & Arbib, 1992; Fitzpatrick et al. 1992a, 1994, 1996; Schieppati & Nardone, 1999; Fitzpatrick, 2003; Masani et al. 2003). In quiet standing, the muscle length is usually poorly correlated with CoM angle (Fig. 3) and when it is correlated, is simply moving in the wrong direction both for short duration, higher frequency sways and long duration, low frequency drift in body position (Fig. 5). The relatively slow increase in tension associated with low-frequency sway (e.g. Fig. 2) is associated with a shortening of the muscle rather than a lengthening and so increased muscle length does not provide a stimulus to increased muscle activity. The muscles do not act as springs. Likewise, central, anticipatory control of muscle spring stiffness (Gatev et al. 1999) fails for the same reason.
Possible mechanisms for producing paradoxical muscle movements
Stretch reflex mechanisms
Stretch reflexes regulate muscle length about an operating point or offset that is determined by drive to the intrafusal fibres (β, γ) (Prochazka, 1996; Maltenfort & Burke, 2003). If the operating point is constant (tonically maintained), reflex activation of the muscle as a result of muscle stretch caused by joint movement will produce increased resistance to stretch but cannot produce paradoxical muscle movements because muscle length will not decrease to less than the operating point. To produce paradoxical changes in muscle length by spindle stretch reflexes it is necessary to change the operating point paradoxically with respect to joint angle.
If a local feedback pathway from the spindle afferents (Ia, II) produced co-activation of extrafusal (α) and intrafusal (β, γ) motor neurones of the parent muscle, then a stretch of the muscle spindle, produced by ankle joint rotation, would cause a reflex change in muscle operating point as well as a reflex increase in muscle activation. Changes in spindle length caused by changing extrafusal drive would be cancelled out by co-activation of intrafusal drive. The precision of cancellation would depend on how faithfully intrafusal drive replicated extrafusal drive and thus spindle stretch could register joint angle, albeit with more noise than if contractile length signalled joint angle.
Thus in principle, a local spindle stretch reflex process could account for the observed paradoxical muscle movements. This is an intriguing possibility, because it means that a low level, local reflex process could convert the intrinsically unstable load into a more stable load. Whether this happens in practice, we do not know. Classically, it is understood that γ motor neurones do not receive Ia excitation (Maltenfort & Burke, 2003). On the other hand, β motor neurones innervate both intra- and extrafusal muscle fibres (Maltenfort & Burke, 2003) though they only provide a small fraction of intrafusal excitation (Prochazka, 1996). In fact, any locally integrated information signalling ankle joint angle, such as a combination of muscle length (Ia, II) and tendon stretch (Ib) could provide the stimulus for a peripheral feedback co-activation of intra- and extrafusal drive which would produce paradoxical muscle movements.
Central co-activation of α and γ drive could also provide the paradoxical muscle movements that are observed. Such co-activation would be commonly regarded as ballistic control of muscle force with reflex servo assistance (Carpenter, 1996). The distinguishing feature of this mechanism, as opposed to the peripheral feedback mechanisms, is that the changes in muscle length could be made independently of changes in ankle joint angle. With the peripheral feedback mechanisms described above, changes in muscle length follow and reflect changes in ankle angle.
Force feedback mechanisms
When the muscle–tendon complex as a whole is stretched during forward sway, tension increases (Fig. 2). Thus possibly, muscle force provides a more useful stimulus to reflex stabilization than spindle stretch. Existing evidence indicates that under postural conditions the Golgi tendon organ usually mediates negative force feedback (Prochazka et al. 1997), thus an increase in tension during forward sway would reduce muscle activation and allow the muscle to lengthen which is the opposite of what is observed. However, there is evidence during fictive locomotion in spinal cats that tendon organ afferent input can be switched into a positive feedback mode during the extensor portion of the step cycle (Guertin et al. 1995) indicating that appropriate spinal circuitry may be available for human standing. Moreover, there are other sources of afferent input that could signal ankle torque. The magnitude and location of pressure between the feet and the ground, as well as deformation of the feet, would signal ankle torque directly, and there is evidence that such cutaneous input helps in the control of upright human balance (Kavounoudias et al. 2001).
If positive force feedback were operating, an increase in muscle tension caused by forward CoM sway would cause the muscle to shorten thereby increasing the stretch of the tendon. Thus, with appropriate gain and no feedback time delay, changes in muscle length could be made to mirror (correlate negatively with) body angle and the muscle–tendon complex as a whole could produce a spring-like change in tension that stabilizes the body.
In our previous publication (Loram et al. 2004) we suggested that paradoxical muscle movements were impossible to produce by stretch reflex mechanisms. As we have shown above, we now believe that paradoxical movements could, in principle, result from stretch reflexes or from positive force feedback. Given that the muscle does actually shorten by tiny amounts as tension increases, the question to be resolved is whether this results from feedback of any kind including positive force feedback (Prochazka et al. 1997) or whether it results from internal model based, anticipatory modulation of muscle activity.
What sort of control process is human standing?
The low stiffness of the tendon and foot means that the muscles and the load (body) are decoupled. For short periods of time, the muscle length can be actively modulated independently of the load. This has two consequences. (i) Muscle length does not slavishly reflect joint angle as has generally been assumed. This suggests that simple feedback systems designed to control muscle length are not useful in standing. (ii) An impulsive predictive mode of control becomes easy and economical. Impulses are generated by transient shifts in muscle length. A temporary stretch of the SEC produces a rise in tension and the tension–time integral defines the impulse. These impulses are repeatedly generated to dynamically maintain balance (Loram & Lakie, 2002a; Lakie et al. 2003). Successful balance is due to the accuracy of individual anticipatory adjustments in bias. These ideas of low joint stiffness and of ballistic, planned, short-duration actions are more commonly associated with the higher level computational processes involving internal models and the cerebellum (Gomi & Kawato, 1996; Morasso et al. 1999; Thoroughman & Shadmehr, 2000; Wolpert & Ghahramani, 2000).
It is possible that reflex reactions have little or no role in quiet standing. For example, careful experimental determination of the forward and feedback transfer functions revealed that the overall reflex loop gain is insufficient to account for stability in standing (Fitzpatrick et al. 1996). This would be in agreement with Evans et al. (1983) who found little evidence for stretch reflexes when ankle movements were less than ± 0.5 deg and would also be in agreement with the lack of reflex response to ankle perturbations which are in the range of ankle rotations encountered in quiet standing (Gurfinkel et al. 1974; Loram & Lakie, 2002b). A consequence of maintaining balance is that by some means, the nervous system produces a muscle length that on average changes in opposition to the CoM angle (Fig. 3). Even allowing no time for neural transmission, integration of a multisensory input and planning, the output of the nervous system must on average be at least 160 ms ahead of body sway (Fig. 4). Proposed mechanisms based on peripheral feedback must explain how this delay is compensated for. An explanation of human balance based on higher level control is perhaps more plausible.
Acknowledgments
We would like to thank The Leverhulme Trust for their support of I.D.L. through this project.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.073437
http://jp.physoc.org/cgi/content/full/jphysiol.2004.073437/DC1 and contains supplementary material consisting of a movie entitled Real time sonograph of standing.
References
- Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature. 1977;265:114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. Neurophysiology. 3rd. London: Arnold; 1996. [Google Scholar]
- Casadio M, Morasso PG, Sanguineti V. Direct measurement of ankle stiffness during quiet standing: implications for control modelling and clinical application. Gait Posture. 2005 doi: 10.1016/j.gaitpost.2004.05.005. in press. [DOI] [PubMed] [Google Scholar]
- Evans CM, Fellows SJ, Rack PM, Ross HF, Walters DK. Response of the normal human ankle joint to imposed sinusoidal movements. J Physiol. 1983;344:483–502. doi: 10.1113/jphysiol.1983.sp014953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC. More pulsating movement. J Physiol. 2003;551:4. doi: 10.1113/jphysiol.2003.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Gorman RB, Burke D, Gandevia SC. Postural proprioceptive reflexes in standing human subjects: bandwidth of response and transmission characteristics. J Physiol. 1992a;458:69–83. doi: 10.1113/jphysiol.1992.sp019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK, McCloskey DI. Stable human standing with lower-limb muscle afferents providing the only sensory input. J Physiol. 1994;480:395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Taylor JL, McCloskey DI. Ankle stiffness of standing humans in response to imperceptible perturbation: reflex and task-dependent components. J Physiol. 1992b;454:533–547. doi: 10.1113/jphysiol.1992.sp019278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc R Soc Lond B Biol Sci. 2001;268:229–233. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514:915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi H, Kawato M. Equilibrium-point control hypothesis examined by measured arm stiffness during multijoint movement. Science. 1996;272:117–120. doi: 10.1126/science.272.5258.117. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group-I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfinkel VS, Ivanenko YP, Levik YS, Babakova IA. Kinesthetic reference for human orthograde posture. Neuroscience. 1995;68:229–243. doi: 10.1016/0306-4522(95)00136-7. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Lipshits MI, Popov KY. Is the stretch reflex the main mechanism in the system of regulation of the vertical posture of man? Biophysics. 1974;19:761–766. [PubMed] [Google Scholar]
- Herbert RD, Moseley AM, Butler JE, Gandevia SC. Change in length of relaxed muscle fascicles and tendons with knee and ankle movement in humans. J Physiol. 2002;539:637–645. doi: 10.1113/jphysiol.2001.012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, MacPherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. Oxford: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- Houk JC, Rymer WZ. Neural control of muscle length and tension. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology, Section 1, The Nervous System. Bethesda, MD, USA: American Physiological Society; 1981. pp. 257–323. [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney RE, Hunter IW. Dynamics of human ankle stiffness – variation with displacement amplitude. J Biomech. 1982;15:753–756. doi: 10.1016/0021-9290(82)90090-2. [DOI] [PubMed] [Google Scholar]
- Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM. The spring in the arch of the human foot. Nature. 1987;325:147–149. doi: 10.1038/325147a0. [DOI] [PubMed] [Google Scholar]
- Lakie M, Caplan N, Loram ID. Human balancing of an inverted pendulum with a compliant linkage: neural control by anticipatory intermittent bias. J Physiol. 2003;551:357–370. doi: 10.1113/jphysiol.2002.036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Human balancing of an inverted pendulum: position control by small, ballistic-like, throw and catch movements. J Physiol. 2002a;540:1111–1124. doi: 10.1113/jphysiol.2001.013077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol. 2002b;545:1041–1053. doi: 10.1113/jphysiol.2002.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Paradoxical muscle movement in human standing. J Physiol. 2004;556:683–689. doi: 10.1113/jphysiol.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Changes in Achilles tendon moment arm from rest to maximum isometric plantarflexion: in vivo observations in man. J Physiol. 1998;510:977–985. doi: 10.1111/j.1469-7793.1998.977bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus R. The Croonian Lecture: animal posture. Proc R Soc Lond B Biol Sci. 1925;98:339–353. [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Maltenfort MG, Burke RE. Spindle model responsive to mixed fusimotor inputs and testable predictions of beta feedback effects. J Neurophysiol. 2003;89:2797–2809. doi: 10.1152/jn.00942.2002. [DOI] [PubMed] [Google Scholar]
- Masani K, Popovic MR, Nakazawa K, Kouzaki M, Nozaki D. Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. J Neurophysiol. 2003;90:3774–3782. doi: 10.1152/jn.00730.2002. [DOI] [PubMed] [Google Scholar]
- Massion J, Alexandrov A, Frolov A. Why and how are posture and movement coordinated? Brain Mechanisms for Integration Posture Movement Prog Brain Res. 2004;143:13–27. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Baratto L, Capra R, Spada G. Internal models in the control of posture. Neural Netw. 1999;12:1173–1180. doi: 10.1016/s0893-6080(99)00058-1. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Sanguineti V. Ankle muscle stiffness alone cannot stabilize balance during quiet standing. J Neurophysiol. 2002;88:2157–2162. doi: 10.1152/jn.2002.88.4.2157. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Schieppati M. Can muscle stiffness alone stabilize upright standing? J Neurophysiol. 1999;82:1622–1626. doi: 10.1152/jn.1999.82.3.1622. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Bizzi E. Motor learning through the combination of primitives. Philos Trans R Soc B Biol Sci. 2000;355:1755–1769. doi: 10.1098/rstb.2000.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1976;26:59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Neilson PD, Neilson MD. The role of action reflexes in the damping of mechanical oscillations. Brain Res. 1978;142:439–453. doi: 10.1016/0006-8993(78)90907-1. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. Proprioceptive feedback and movement regulation; pp. 89–127. [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. Implications of positive feedback in the control of movement. J Neurophysiol. 1997;77:3237–3251. doi: 10.1152/jn.1997.77.6.3237. [DOI] [PubMed] [Google Scholar]
- Rack PMH. Stretch reflexes in man: the significance of tendon compliance. In: Barnes W, Gladden M, editors. Feedback and Motor Control in Invertebrates and Vertebrates. London: Croom-Helm Ltd; 1985. [Google Scholar]
- Rack PM, Ross HF, Thilmann AF, Walters DK. Reflex responses at the human ankle: the importance of tendon compliance. J Physiol. 1983;344:503–524. doi: 10.1113/jphysiol.1983.sp014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Group II spindle afferent fibers in humans: their possible role in the reflex control of stance. Peripheral and Spinal Mechanisms in the Neural Control of Movement Prog Brain Res. 1999;123:461–472. doi: 10.1016/s0079-6123(08)62882-4. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach J, Gill KF. System Modelling and Control. 3rd. London: Edward Arnold; 1992. [Google Scholar]
- Shadmehr R, Arbib MA. A mathematical-analysis of the force-stiffness characteristics of muscles in control of a single joint system. Biol Cybern. 1992;66:463–477. doi: 10.1007/BF00204111. [DOI] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–747. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Rietdyk S, Ishac M. Ankle muscle stiffness in the control of balance during quiet standing. J Neurophysiol. 2001;85:2630–2633. doi: 10.1152/jn.2001.85.6.2630. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3:1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- Zajac FE, Gordon ME. Determining muscle's force and action in multi-articular movement. Exerc Sport Sci Rev. 1989;17:187–230. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.