Abstract
Sex pheromones are intraspecific chemical signals that are crucial for mate attraction and discrimination. In Drosophila melanogaster, the predominant hydrocarbons on the cuticle of mature female and male flies are radically different and tend to stimulate or inhibit male courtship, respectively. This sexual difference depends largely upon the number of double bonds (one in males and two in females) added by desaturase enzymes. A mutation was caused by a PGal4 transposon inserted in the desat1 gene that codes for the desaturase crucial for setting these double bonds. Homozygous mutant flies produced 70–90% fewer sex pheromones than control flies, and the pheromonal difference between the sexes was almost abolished. A total of 134 excision alleles were induced by pulling out all or a part of the transposon. The pheromonal profile was generally rescued in excision alleles with a completely or largely removed transposon whereas it remained mutant in alleles with a larger piece of the transposon. Five desat1 transcripts were detected during larval-to-adult development. Their levels were precisely quantified in 24-hr-old adults, a critical period for the production of sex pheromones. Three transcripts significantly varied between control females and males; however, the predominant transcript showed no difference. In mutant flies, the predominant transcript was highly decreased with the two sexually dimorphic transcripts.These two transcripts were also absent in the sibling species D. simulans, which shows no sexually dimorphic hydrocarbons. We also induced a larval-lethal allele that lacked all transcripts and failed to complement the defective hydrocarbon phenotype of mutant alleles.
IN many animals, mate choice depends on species-specific signals that are exchanged during courtship (Bradbury and Vehrencamp 1998). Among the different signals involved in communication, chemical messages often play a major role in invertebrates (Wyatt 2003). In Drosophila melanogaster, sexual orientation relies largely upon the hydrocarbons present on the cuticle (cuticular hydrocarbons, or CHs; Jallon 1984; Ferveur et al. 1997). Predominant CHs are sexually dimorphic in both their occurrence and their effects: the male's 7-tricosene (7-T) is thought to stimulate females and to inhibit males while the female's 7,11-dienes (7,11-heptacosadiene, or 7,11-HD, and 7,11-nonacosadiene, or 7,11-ND) moderately stimulate conspecific male courtship (Ferveur and Sureau 1996) and strongly prevent mating by males of two closely related species, D. simulans and D. mauritiana (Coyne et al. 1994; Coyne and Oyama 1995; Savarit et al. 1999). The two latter species show no sexual dimorphism for predominant CHs and both sexes predominantly produce 7-T (Jallon and David 1987; Cobb and Jallon 1990).
In D. melanogaster, the sexual difference for the production of CHs is largely based on the number of double bonds present on the carbon chains. Desaturase enzymes are thus crucial in transforming the fatty acids into mature CHs. In this species, six unique desaturase-encoding sequences have been deduced from the genomic sequence (Knipple et al. 2002). Among these sequences, the two clustered genes desat1 and desat2 code for two closely related desaturases with a different specificity for fatty acid precursors (palmitate and myristate, respectively; Wicker-Thomas et al. 1997; Dallerac et al. 2000). A survey of 24 geographic strains revealed that all strains, except those collected in Africa and in the Caribbean, have a similar mutation in the 5′ region of the desat2 gene. The occurrence of this mutation has been correlated with a variable mating propensity shown by strains with unusual cuticular hydrocarbons and has been interpreted as a case of incipient speciation (Takahashi et al. 2001; Ting et al. 2001; Fang et al. 2002). Unlike desat2, which is predicted to produce only one transcript and which is known to be involved only in introducing a specific set of double bonds (on carbons 5 and 9), that is seen only in a minority of geographical strains, desat1 is active in all D. melanogaster strains and has a complex structure, which suggests that it could play a variety of roles. Its first exon shows five promoting sequences that could code for five transcripts whose translation would result in the same unique desaturase enzyme, perhaps expressed in different tissues (http://flybase.net/).
We carried out the genetic and molecular study of the desat1 gene with a strain that carries a PGal4 transposon inserted in its genome in which the production of sex pheromones was drastically reduced. The remobilization of the transposon allowed us to rescue the CH profile in 75% of alleles; the other alleles induced either a mutant-like or an intermediate profile. In most alleles, a relationship was found between the CH phenotype and the molecular structure of desat1. We detected five desat1 transcripts and measured their variation in females and males of various genotypes and species. We also induced a “larval lethal” null allele that produced no transcript and failed to complement the mutant CH phenotype.
MATERIALS AND METHODS
Stocks and genetic procedure:
All D. melanogaster and D. simulans strains were raised on yeast/cornmeal/agar medium and kept at 25° on a 12:12 hr light/dark cycle. Canton-S (Cs) was used as the control strain for the production of D. melanogaster male and female CHs (Figure 1). The D. simulans strain, collected in the Seychelles archipelago, has been for maintained >20 years in the lab (Ferveur 1991). Crosses were performed using standard techniques. A description of the chromosomes and genetic tools used in this study can be found in Lindsley and Zimm (1992). To generate derivative lines of desat11573-1, we used the scheme described by Cooley et al. (1988) to mobilize the PGal4 transposon. Excision of the transposon was performed by crossing homozygous desat11573-1 females with males from a jump starter strain, which provided the P-element transposase (Robertson et al. 1988). Male progeny carrying the desat11573-1 PGal4 transposon and the transposase-producing Δ2-3 chromosome were crossed to w; +/TM3 females. To get flies without the P transposon, males of the next generation were scored for the loss of the mini-white+ gene. Each excision event (desat11573-exc) was subsequently amplified in separate strains by crossing each w−; desat11573-exc /TM3 male with several w−; PGal4-w+/TM3 virgins. Within each strain, white eye (w−; desat11573-exc/TM3) males and females were mated together to produce a stable line.
Figure 1.
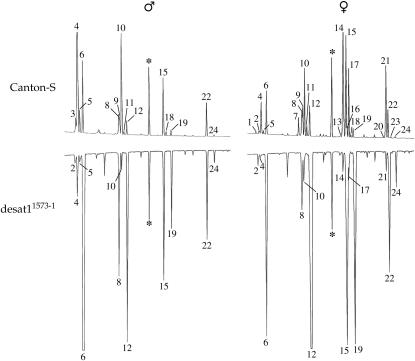
Representative mirrored gas chromatograms of hexane extracts of individual male and female flies of the control (Canton-S) and mutant desat11573-1 strains. Each peak corresponds to a single hydrocarbon whose identity was previously revealed by mass spectrometry. All identified male and female CH peaks are numbered sequentially by increasing chain length (in italics); key to numbers is as follows. 23 carbons, (1) 7,11-tricosadiene (7,11-TD); (2) 2-methyldocosane (23Br); (3) 9-tricosene (9-T); (4) 7-tricosene (7-T); (5) 5-tricosene (5-T); (6) n-tricosane (23Lin). 25 carbons, (7) 7,11-pentacosadiene (7,11-PD); (8) 2-methyltetracosane (25Br); (9) 9-pentacosene (9-P); (10) 7-P; (11) 5-P; (12) n-pentacosane (25Lin). 27 carbons, (13) 9,13-HD; (14) 7,11-HD; (15) 2-methylhexacosane (27Br); (16) 5,9-HD; (17) 7-heptacosene (7-H); (18) 5-H; (19) n-heptacosane (27Lin). 29 carbons, (20) 9,13-ND; (21) 7,11-ND; (22) 2-methyloctacosane (29Br); (23) 9-nonacosene (9-N); (24) n-nonacosane (29Lin). The chromatograms are to the same scale: they were aligned and calibrated by using an added standard of hexacosane (*).
Four Df(3R) deficiencies that theoretically partially or totally delete the desat1 gene [T32 (86E2-87C7), P21 (86E19F1-87B11-15), kar-H10 (87A7-87D2), and ry615 (87B11-15-87E8-11); Gausz et al. 1981; Garcia-Bellido et al. 1983; Reuter et al. 1987] were available. All deficiencies were tested in reciprocal complementation crosses with the C′1 null allele to measure their effect on the CH phenotype, developmental lethality, and desat1 transcription profile. To assess the developmental stage during which C′1/C′1 and C′1/ry615 died, the proportion of first, second, and third instar surviving larvae was compared with that of heterozygotes (C′1/TM3; ry615/TM3) every 24 hr at 25°. The genotype of larvae was assessed by the presence/absence of the TM3, Ser Act5-GFP balancer chromosome that induces a characteristic fluorescent pattern (Ferrandon et al. 1998). The l(3) S028813 allele with the P(w) transposon (Deak et al. 1997), which moderately affected the CH phenotype (Labeur et al. 2002), was also tested in complementation with the C′1 allele.
Extraction and analyses of CHs:
Male and female flies were sexed 0–2 hr after emergence under light CO2 anesthesia and aged, in groups of five, in standard food vials. CHs were extracted from 5-day-old individual flies by gas chromatography following hexane extraction according to the method of Pechiné et al. (1985) and modified by Ferveur (1991). Analyses were performed with a Varian CP3380 chromatograph and equipped with a Cp-sil 25 mx 0.25-mm capillary column, using hydrogen as the carrier gas. The temperature program was: 120°–140°, 10°/min; 140°–300°, 4°/min, and then a constant temperature of 300° during 28 min. Peak detection was carried out using a flame ionization detector that yielded retention times and areas under each peak. All the main D. melanogaster CHs have been identified and characterized (Antony and Jallon 1982; Pechiné et al. 1985, 1988; Jallon and Pechiné 1989). Twenty-four CHs were systematically detected in female flies and 14 in male flies, both with chain lengths ranging from 23 to 29 carbons. Each CH was characterized by its percentage relative to the total amount of CHs. In addition, the area of each CH peak was compared with the area of an internal hexacosane standard to calculate its absolute amount (in nanograms). Our chemical analysis focused on 7,11-dienes (7,11-HD and 7,11-ND), which are the predominant CHs of mature wild-type D. melanogaster females, and on 7-monoenes [7-T and 7-pentacosene (7-P)], which are abundant in wild-type D. melanogaster males and in D. simulans flies. We also measured the sum of all detected unsaturated CH (ΣDesat) and each of the saturated CHs (23Lin, 25Lin, 27Lin, 29Lin), which are predominant in mutant flies, together with their sum (ΣLin). To compare the effect of desat alleles, we designed the desaturation index (DI) whose formula is: DI = (ΣDesat − ΣLin)/(ΣDesat + ΣLin). DI can vary between +1 (for flies with high ΣDesat and low ΣLin) and −1 (for flies with low ΣDesat and high ΣLin). However, as in other studies, 5,9-HD was confounded with methyl-hexacosane (27Br; Coyne et al. 1999), and the sum of these two compounds seemed to not vary between mutant and control females.
Molecular characterization:
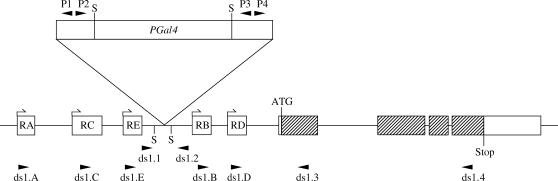
To localize the P-element insertion, flanking DNA sequences were determined by sequencing inverse PCR products as described in detail at http://www.fruitfly.org/p_disrupt/inverse_pcr.html. In brief, genomic DNA was prepared from 50 adult flies, purified according to the method described in Sambrook et al. (1989), digested with Sau3A1 (Promega, Madison, WI), and then ligated under dilute conditions (1/40°) to increase intramolecular ligation. Four oligonucleotide primers internal to the transposon (P1: AGTCGGCAAATATCGCATGCTTGTTC; P2: TGCCGTCACAGATAGATTGGCTTCAG; P3: CCTTAGCATGTCCGTGGGGTTTGAAT; P4: CTTGCCGACGGGACCACCTTATGTTATT; Figure 2) were used with Taq polymerase (Eppendorf, Madison, WI). Thermal cycling was: 3 min at 94°; 40 cycles of 94° for 45 sec (denaturation), 60° for 45 sec (reannealing), 72° for 1 min/kb (extension); and a 10-min terminal extension at 72°. PCR products were purified and sequenced bidirectionally (MWG-Biotech, Ebersberg, Germany). To identify the insertion site of the P element in desat11573-1, BLASTN was used to align the sequences of PCR products and the available genomic DNA sequence on the BDGP database (http://flybase.net/blast/).
Figure 2.
Schematic of the desat11573-1 locus (chromosome III, 87B10-11). The triangle represents the PGal4 (PGawB) transposon, which is inserted in the first intron of the desat1 gene with its 3′ extremity mapped at −1691 bp from the ATG codon. The five open boxes below the triangle represent the five specific exons for each transcript (RA, RC, RE, RB, and RD), and the hatched boxes represent the translated desat1 region. The primers used are indicated by solid arrowheads (see materials and methods). Briefly, P1/P2 and P3/P4, respectively, were used to amplify the 5′ and 3′ flanking DNA region after Sau3A1 restriction (sites are noted by “S”); ds1.1/P1 and P4/ds1.2 were used to obtain ∼1 kb of 5′ and 3′ flanking DNA; and ds1.A/ds1.3 and ds1.A/ds1.4, respectively, were used to detect the RA transcript in Q-PCR and RT-PCR (and similarly with the four other ds1.C, E, B, and D primers used to detect their respective RC, RE, RB, and RD transcripts).
Characterization of ∼1 kb DNA flanking 5′ and 3′ the insertion of various desat11573-exc alleles was carried out by PCR on genomic DNA with the primers P1/ds1.1 (CTTCTTTCGTGCATTTTAACTAAGC) and P4/ds1.2 (GGTTCTTCGTCGTCGGCAAT), respectively. Following amplification, one-fifth of the PCR product was analyzed by electrophoresis on a 1% (w/v) agarose gel containing ethidium bromide.
Total RNA was isolated from frozen adult flies or larvae using Trizol reagent (Invitrogen, San Diego). For RT-PCR, 1 μg of total RNA was reverse transcribed in 20 μl using SuperScript II RNase H minus reverse transcriptase (from M-MLV of Escherichia coli; Invitrogen) in the presence of oligo(dT) primer according to the manufacturer's instructions. Then 1 μl of cDNA was amplified with 2 μm of a primer specific for each transcript (for RA, ds1.A: GCCATCACTAAACCAGGAGAATA; for RC, ds1.C: CGCCACTCCTACACTCAAAAATA; for RE, ds1.E: CAGATACAACATCCTAAACAAATCG; for RB, ds1.B: TAATGGCCCCATCCTGGT; for RD, ds1.D: CGAAACGGCTTGTTAATTTCTAGC) and a common primer in the desat1 coding region (ds1.4: GGAGAGGGATGGCATAACTACCATC), following the procedure described above (Figure 2). Two primers corresponding to the desat2 gene, as described in Dallerac et al. (2000), were used to detect the desat2 transcript.
For the real-time PCR assay (Q-PCR), the procedure was similar to that described for RT-PCR except that the reactions were done on 1 μl of cDNA (made from 2 μg of total RNA) with the Quantitect SybrGreen kit (QIAGEN, Chatsworth, CA) with 2 μm of each specific primer (ds1.A-E) combined with ds1.3 primer (TTCGAGTGCGATGTGGAAACCACCG) in a final volume of 25 μl. These primers were designed to produce fragments that were similar in size (212–291 bp) to the Actin5C control (215 bp; see below) to quantify them accurately. Cycling conditions were as follows: 15 min at 95°, followed by 40 cycles of 94° for 45 sec, 60° for 30 sec, and 72° for 30 sec. For all reactions, each sample was duplicated on 96-well plates with optical sealing tape, and signals were measured with sequence detector system software (Applied Biosystems, Courtaboeuf, France). All signal thresholds to be compared were standardized with the Actin 5C mRNA, using an ABI prism 7700 detector system (Applied Biosystems). For both RT- and Q-PCR assays, the amplification of Actin 5C (Act5C.dir: CAGATCATGTTCGAGACCTTCAA and Act 5C.rev: ATCTTCATCAGGTAGTCGGTCAA) served as a control.
Statistics:
The hydrocarbon profiles of various desat11573-exc excision alleles were compared with an ANOVA, using their DIs. desat11573-exc alleles were considered as mutant, rescue, or intermediate according to the difference between their DI and the DIs of the mutant 1573-1 and/or of control Cs flies. The levels of desat1 transcripts were compared between the sexes (with a Mann-Whitney test) and among genotypes (with a Kruskal-Wallis test). Only P-values <0.05 were considered to be statistically significant.
RESULTS
The production of sex pheromones is drastically decreased in mutant flies:
We initially analyzed by gas chromatography the CHs in mature D. melanogaster female and male flies of >600 PGal4 strains obtained from several labs and found one strain (no. 1573) with defective CH profiles in both sexes. Homozygous mutant flies were perfectly viable and fertile but their production of unsaturated CHs with one and two double bonds (respectively, monoenes and dienes), including the known sex pheromones of control flies, was substantially smaller than that of controls (Figure 1; Table 1). For example, control Cs females produced 675 ng of 7,11-dienes [including the predominant 7,11-HD (peak 14) and 7,11-ND (peak 21)], representing 37.8% of the SumCH, while mutant females produced only 71 ng of 7,11-dienes (2.6% of the SumCH). Mutant females also decreased their levels of all other unsaturated CHs, including 7-T (peak 4), and 7-P (peak 10) if compared to control females (7-T + 7-P = 72 and 165 ng, respectively). The total fraction of unsaturated CHs dropped from 62.7% in control females to 6.2% in mutant females. Mutant males also showed a drastic decrease of their overall level of unsaturated CHs (7.2%) if compared to that of control males (62.7%). In particular, the absolute quantity of 7-monoenes (7-T + 7-P) in control males (512 ng = 52.4%) was reduced in mutant males (157 ng = 5.6%).
TABLE 1.
Amounts of CHs on 5-day-old D. melanogaster males and females of the control Cs and mutant (1573) strains
| Peak no.
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 7 | 8 | 9 | 10 | 12 | 14 | 15 | 16 | 17 | 19 | 21 | 22 | 24 | |||
| 23Br | 7T | 23Lin | 7,11PD | 25Br | 9P | 7P | 25Lin | 7,11HD | 27Br | + | 5,9HD | 7H | 27Lin | 7,11ND | 29Br | 29Lin | SumCH | |
| Cs female | ||||||||||||||||||
| ng | 25 (9) | 51 (9) | 98 (11) | 35 (5) | 85 (11) | 65 (9) | 114 (12) | 79 (9) | 441 (43) | 331 (34) | 141 (20) | 24 (4) | 189 (36) | 51 (10) | 3 (1) | 1788 (181) | ||
| % | 1.4 (0.6) | 3.0 (0.4) | 5.5 (0.4) | 2.0 (0.2) | 4.7 (0.4) | 3.6 (0.4) | 6.5 (0.5) | 4.3 (0.3) | 25.2 (1.8) | 18.9 (1.2) | 7.9 (0.7) | 1.3 (0.1) | 10.0 (1.1) | 2.7 (0.3) | 0.2 (0.1) | |||
| 1573 female | ||||||||||||||||||
| ng | 7 (2) | 12 (2) | 457 (29) | 1 (0) | 122 (9) | 0 (0) | 63 (5) | 864 (41) | 32 (4) | 487 (25) | 2 (1) | 378 (19) | 20 (3) | 174 (8) | 29 (2) | 2681 (89) | ||
| % | 0.3 (0.1) | 0.5 (0.1) | 17.0 (0.8) | 0.0 (0) | 4.5 (0.3) | 0.0 (0) | 2.3 (0.2) | 32.1 (0.7) | 1.2 (0.1) | 18.3 (0.8) | 0.1 (0) | 14.2 (0.7) | 0.8 (0.1) | 6.6 (0.4) | 1.1 (0.1) | |||
| 3 | 4 | 5 | 6 | 8 | 9 | 10 | 11 | 12 | 15 | 17 | 19 | 22 | 24 | |||||
|
|
9T
|
7T
|
5T
|
23Lin
|
25Br
|
9P
|
7P
|
5P
|
25Lin
|
27Br
|
|
7H
|
27Lin
|
29Br
|
29Lin
|
SumCH
|
||
| Cs male | ||||||||||||||||||
| ng | 33 (6) | 376 (50) | 33 (5) | 136 (18) | 47 (12) | 27 (8) | 136 (12) | 4 (1) | 23 (3) | 113 (14) | 4 (1) | 8 (1) | 39 (5) | 2 (0) | 982 (81) | |||
| % | 3.2 (0.3) | 37.4 (2.7) | 3.3 (0.3) | 13.7 (1.1) | 4.4 (0.9) | 3.0 (0.8) | 15.0 (1.9) | 0.4 (0.1) | 2.4 (0.2) | 11.7 (1.1) | 0.4 (0.1) | 0.9 (0.1) | 4.1 (0.4) | 0.2 (0.1) | ||||
| 1573 male | ||||||||||||||||||
| ng | 18 (3) | 132 (13) | 21 (2) | 1536 (108) | 300 (20) | 5 (2) | 39 (4) | 1 (0) | 410 (18) | 294 (15) | 0 (0) | 123 (8) | 139 (7) | 16 (1) | 3033 (165) | |||
| % | 0.6 (0.1) | 4.3 (0.4) | 0.7 (0.1) | 50.0 (1.1) | 9.9 (0.4) | 0.2 (0.1) | 1.3 (0.1) | 0.0 (0) | 13.7 (0.5) | 9.8 (0.4) | 0.0 (0) | 4.1 (0.3) | 4.7 (0.2) | 0.5 (0.1) | ||||
Data shown are the mean absolute amount (given in nanograms ±SEM) and the percentage relative to the overall amount of detected CHs (SumCH) for N = 20 flies. For the sake of clarity, we show only the most abundant CHs. For the nomenclature and identity of all CHs, refer to Figure 1.
Conversely, mutant flies of both sexes showed much higher levels of n-alkanes than control flies (Figure 1; Table 1). In mutant females and males, the sum of n-alkanes (23Lin + 25Lin + 27Lin +29Lin = ΣLin) represented 60.5% and 67.7% of the detected CHs, respectively (vs. 11.1% and 17% in Cs females and males). 25Lin (n-pentacosane; peak 12) was the predominant CH of mutant females (31.6% = 837 ng), and 23Lin (n-tricosane; peak 6) was the predominant CH (50.2%) of mutant males and was 10 times more abundant (1387 ng) than in Cs males (136 ng). Also, the overall amount of CHs (SumCH) increased highly in mutant males (+176%) and females (+48%) if compared to same-sex control flies (Table 1). This increase was mainly caused by linear alkanes but not by methyl-alkanes, whose relative abundance increased only slightly in mutant females (+2%) and males (+3.9%). The DI (materials and methods), which can oscillate between +1 (for high production of unsaturated CHs) and −1 (for low production of unsaturated CHs) was very different between Cs flies (+0.688 in females and +0.566 in males) and mutant flies (−0.830 and −0.811, respectively).
The PGal4 mutation induces a quasi-recessive effect on the CH profile because heterozygous mutant flies showed only a moderate variation. The percentage of alkenes decreased by 20–30%, and the percentage of alkanes increased in the same proportion in heterozygous flies, if compared to control flies. Their SumCH was similar to that of control flies (data not shown).
The transposon that decreases pheromonal production is inserted in the desat1 gene:
First, we verified with a Southern blot that only one PGal4 transposon was inserted in the genome of the 1573-1 enhancer-trap strain (data not shown).
Then the gene altered by the transposon was mapped. After the digestion and circularization of the DNA of the mutant strain, primers were designed to amplify the genomic regions flanking the insertion point (Figure 2; see materials and methods). Two fragments, one in 5′ and the other in 3′ of the transposon, were cloned and sequenced. The comparison with the BDGP database revealed that both fragments share a complete identity with two contiguous sequences of the desat1 gene, located on chromosome III at 87B10-11. desat1 is a gene that codes for a Δ9 desaturase enzyme involved in setting a double bond on the carbon ω7 of fatty acids, leading to unsaturated CHs like 7-monoenes and 7,11-dienes (Wicker-Thomas et al. 1997). Therefore, the altered CH phenotypes shown by homozygous mutant female and male flies are probably the result of a mutation in desat1.
The transposon inserted in the 1573-PGal4 allele (now named desat11573-1, or 1573-1, in this article) was pulled out to create 134 new excision alleles (1573-exc). All excision alleles produced homozygous viable female and male flies, with the exception of the 1573-C′1 and -O7 alleles. We compared the DIs produced by homozygous 1573-exc flies of both sexes with the DIs of Cs and 1573-1 strains (Table 2). According to this criterion, a phenotype similar to that of wild-type flies was rescued for 110 excision alleles in females (with +0.674 ≥ DI ≥ +0.217) and for 117 alleles in males (+0.730 ≥ DI ≥ +0.296). This rescue demonstrates that the PGal4 transposon inserted in desat1 causes the mutant CH phenotype. Fourteen other alleles induced a mutant CH profile in both females (−0.882 ≤ DI ≤ −0.542) and males (−0.894 ≤ DI ≤ −0.711). With DI values significantly different from both control and mutant profiles, 8 alleles were considered as intermediate in females (−0.389 ≤ DI ≤ +0.075), whereas only 1 allele was considered intermediate in males (H6; DI = +0.171).
TABLE 2.
Principal CH parameters of 5-day-old male and female flies homozygous for various desat11573-1 alleles and the size of the transposon inserted into desat1
| Male
|
Female
|
Transposon length (kb)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DI | ΣDesat (%) | ΣLin (%) | SumCH | DI | ΣDesat (%) | ΣLin (%) | SumCH | |||
| Wild-type: | Cs | +0.566 (0.041) | 62.7 (2.2) | 17.2 (1.4) | 982 (81) | +0.687 (0.017) | 61.0 (1.0) | 11.3 (0.6) | 1788 (181) | |
| Mutant: | 1573 | −0.811 (0.010) | 7.1 (0.4) | 68.5 (0.8) | 3033 (165) | −0.844 (0.010) | 5.4 (0.3) | 64.3 (1.1) | 2681 (89) | 11.3 |
| Excision-mutant | ||||||||||
| E1 | −0.894 (0.010) | 3.9 (0.4) | 70.1 (0.6) | 2921 (73) | −0.828 (0.020) | 5.4 (0.6) | 57.5 (1.2) | 2023 (120) | 6.2 | |
| J2 | −0.891 (0.011) | 4.0 (0.4) | 68.9 (1.0) | 2366 (244) | −0.763 (0.013) | 7.8 (0.4) | 58.4 (0.9) | 2282 (70) | 8.7 | |
| F3 | −0.880 (0.008) | 4.2 (0.2) | 66.6 (1.4) | 2471 (108) | −0.866 (0.003) | 4.5 (0.1) | 63.0 (0.4) | 1804 (251) | 9.6 | |
| P3 | −0.873 (0.029) | 4.7 (1.1) | 69.7 (1.7) | 2481 (59) | −0.743 (0.012) | 9.1 (0.5) | 62.0 (0.9) | 2029 (57) | 10.6 | |
| P′2 | −0.872 (0.008) | 4.7 (0.3) | 69.1 (0.4) | 1760 (219) | −0.752 (0.011) | 8.4 (0.6) | 58.8 (1.0) | 2219 (62) | 10.3 | |
| C1 | −0.863 (0.013) | 5.2 (0.5) | 70.7 (0.7) | 2897 (176) | −0.872 (0.005) | 4.6 (0.2) | 67.3 (0.2) | 2086 (190) | 10.6 | |
| C6 | −0.862 (0.025) | 5.1 (1.1) | 68.5 (0.6) | 2695 (194) | −0.660 (0.060) | 11.6 (2.0) | 56.6 (2.6) | 2459 (37) | 8.1 | |
| C2 | −0.855 (0.012) | 5.8 (0.4) | 73.7 (1.0) | 2713 (248) | −0.882 (0.015) | 4.2 (0.5) | 67.8 (1.0) | 1880 (36) | 10.6 | |
| C3 | −0.849 (0.018) | 5.9 (0.6) | 72.0 (1.4) | 2524 (50) | −0.696 (0.025) | 10.3 (0.8) | 57.5 (0.8) | 2090 (147) | 8.1 | |
| A4 | −0.844 (0.015) | 6.1 (0.5) | 72.6 (1.3) | 2726 (170) | −0.821 (0.014) | 5.9 (0.4) | 60.4 (0.8) | 2136 (27) | 11.0 | |
| D1 | −0.824 (0.010) | 7.0 (0.3) | 72.4 (1.2) | 1825 (174) | −0.823 (0.014) | 6.3 (0.6) | 64.9 (0.4) | 2296 (105) | 9.7 | |
| C5 | −0.817 (0.008) | 7.0 (0.4) | 69.2 (2.6) | 2798 (245) | −0.542 (0.040) | 15.9 (1.2) | 53.7 (2.1) | 2551 (119) | 6.9 | |
| P2 | −0.779 (0.008) | 8.4 (0.3) | 68.2 (0.5) | 3051 (202) | −0.819 (0.014) | 6.3 (0.5) | 63.5 (0.9) | 2376 (210) | 7.7 | |
| Q′1 | −0.711 (0.039) | 10.9 (1.3) | 64.5 (3.2) | 2266 (240) | −0.605 (0.006) | 13.1 (0.6) | 52.0 (1.6) | 2122 (135) | 6.4 | |
| Excision-intermediate | ||||||||||
| H6 | +0.171 (0.030) | 41.8 (1.3) | 29.5 (1.1) | 1278 (101) | −0.112 (0.055) | 32.8 (2.0) | 41.1 (2.1) | 1873 (83) | 8.6 | |
| L6 | +0.296 (0.026) | 51.1 (1.1) | 27.8 (1.2) | 1508 (98) | +0.053 (0.045) | 36.8 (1.6) | 33.2 (1.6) | 1969 (64) | 5.1 | |
| F7 | +0.391 (0.070) | 48.5 (2.7) | 21.1 (2.4) | 1467 (67) | −0.389 (0.087) | 22.5 (3.2) | 52.0 (3.7) | 1561 (92) | 0.1 | |
| F4 | +0.395 (0.032) | 55.2 (1.3) | 24.0 (1.4) | 1327 (59) | −0.089 (0.051) | 33.2 (1.6) | 40.2 (2.3) | 1884 (73) | 0.1 | |
| M3 | +0.399 (0.097) | 56.1 (3.0) | 24.0 (2.7) | 1452 (97) | −0.070 (0.038) | 33.4 (1.1) | 38.7 (1.8) | 1636 (47) | 6.8 | |
| L2 | +0.429 (0.018) | 52.4 (1.1) | 20.9 (0.6) | 1373 (52) | +0.073 (0.041) | 36.0 (1.3) | 31.3 (1.5) | 1496 (46) | 5.8 | |
| F6 | +0.446 (0.066) | 59.0 (2.6) | 22.9 (2.8) | 1690 (93) | −0.048 (0.070) | 32.8 (2.2) | 36.7 (2.7) | 1925 (113) | 0.1 | |
| F2 | +0.452 (0.025) | 56.1 (1.3) | 21.1 (0.9) | 1434 (76) | +0.075 (0.048) | 37.1 (1.7) | 32.0 (1.9) | 1812 (127) | 0.1 | |
| Excision-rescue | ||||||||||
| N2 | +0.594 (0.025) | 62.1 (0.9) | 15.9 (1.1) | 1284 (63) | +0.475 (0.047) | 50.5 (1.6) | 18.0 (1.7) | 1604 (67) | 0 | |
| L3 | +0.644 (0.026) | 62.9 (1.0) | 13.7 (1.1) | 1070 (237) | +0.457 (0.022) | 48.6 (1.0) | 18.1 (0.8) | 1475 (38) | 0 | |
| A′2 | +0.676 (0.023) | 61.8 (1.9) | 11.9 (0.8) | 1141 (201) | +0.539 (0.016) | 52.7 (1.0) | 15.8 (0.4) | 1437 (57) | 0 | |
All CH data are mean (±SEM) for N = 10 flies. Excision alleles were classified into three categories according to their DI (see materials and methods). Within each category, alleles were ranked from top to bottom according to male DIs. In mutant alleles (Excision-mutant) DIs were different from that of the control (Cs) and similar to that of mutant 1573-1. DIs of rescued alleles (Excision-rescue) were similar to that of Cs and different from that of 1573-1. DIs of intermediate alleles (Excision-intermediate) were different from the DIs of both Cs and 1573-1, either for both sexes (H6) or for only females (L6–F2). ΣDesat (%) corresponds to the total percentage of all unsaturated CHs relative to the sum of detected CHs (SumCH, given in nanograms); ΣLin (%) corresponds to the total percentage of all saturated linear CHs. The transposon length (shown at the right) indicates the estimated size (in kilobases) of the fragment of the transposon that remains inserted in each allele. This estimation corresponds to the pooled size of three sequences detected by Southern blots.
The total amount of unsaturated CHs (ΣDesat) was much lower in mutant alleles (180 ng for females and 149 ng for males) than in rescued alleles (758 and 678 ng, respectively). Conversely, mutant alleles showed a much higher sum of linear saturated CHs (ΣLin; 1304 and 1778 ng in females and males) than rescued alleles (161 and 235 ng). The SumCH also increased in mutant alleles (2168 and 2535 ng) if compared to rescued alleles (1440 and 1116 ng). The intermediate alleles showed CH levels between those of the control Cs and the mutant 1573-1 strains.
Relationship between the molecular structure of desat1 and the CH phenotype:
We looked for a relationship between the molecular structure of the desat1 locus and the CH profile variation. First, the flanking DNA 5′ and 3′ of the insertion site was amplified with primers designed within the transposon and at 1 kb of the insertion site. This study, carried out with all alleles, revealed no alteration of the desat1 gene, with the exception of the C′1 lethal allele (see below).
When a part of the transposon remained inserted, both the size and the nature of the fragment were determined. For all alleles, each of the three principal sequences (Gal4, miniwhite, and pBSK) forming the transposon was probed with Southern blotting and its size was evaluated by comparison with the complete sequence detected in the 1573-PGal4 strain (data not shown). Then the overall size of the inserted fragment was estimated after pooling the size of the remaining sequences corresponding to the three fragments (Table 2; right column).
In mutant alleles, the size of transposon varied between 11 and 6.2 kb. In intermediate alleles, some fragments were relatively large (5.1–8.6 kb, the largest in H6), whereas others were as small as 155 bp in F2, F4, F6, and F7 (the sequenced fragment was strictly identical in these 4 alleles and corresponded to the feet of the transposon). In most rescued alleles (91), no trace of the transposon was detected, and at least six alleles that were sequenced showed a precise excision. Among these precisely excised alleles, N2 was used as a control in subsequent experiments. In other rescued alleles, a fragment of a significant size remained inserted and either was ≤2 kb (in 14 alleles) or varied between 3 and 6.3 kb (in 9 alleles). No relationship was found between the gravity of the CH phenotype and the nature of the molecular sequence(s) of the transposon fragment that remained in desat1 (data not shown).
The level of desat1 transcripts can vary between genotypes and species:
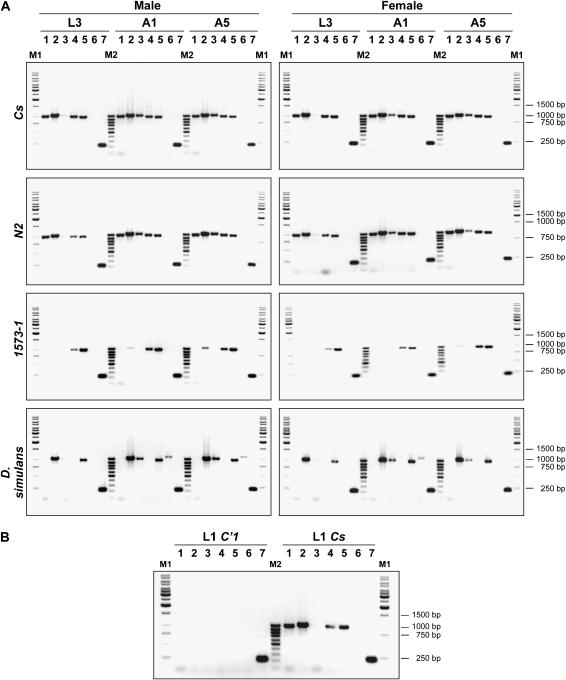
On the basis of its sequence, desat1 was predicted to code for five transcripts, each one with a specific region in the 5′-UTR of desat1 and a common coding region (Figure 2). We first detected these five transcripts with RT-PCR and compared the transcription profile of females and males in the wild-type Cs strain, in homozygotes for the clean excision 1573-N2 allele, and in the mutant 1573-1 allele. The presence and the relative abundance of the five transcripts were measured at four developmental stages: in first and third instar larvae (L1, L3) and in 1-day- and 5-day-old adults (A1, A5; Figure 3).
Figure 3.
Comparison of the desat1 transcription pattern among sexes, genotypes, and species during development. Each transcript was detected by RT-PCR with the primers described in Figure 2. (A) The comparison was carried out between males and females at the following stages: third instar larva (L3), 24-hr-old adult (A1), and 5-day-old adult (A5). (B) Both sexes were pooled in first instar larva (L1). For D. melanogaster, the following genotypes were compared: wild-type Cs and homozygotes for the 1573-N2 rescued excision allele (N2), the 1573-1 mutant allele (1573-1), or the C′1 null allele (C′1). D. simulans is a wild-type strain. Five desat1 transcripts were detected: RA (1), RC (2), RE (3), RB (4), and RD (5). The desat2 transcript (6) and the Actin5C transcript (7) were used as internal markers. M1 and M2 are the molecular weight markers (MBI, Fermentas; 1 kb and 100 bp, respectively). The approximate size of the fragments is indicated on the right.
In Cs and N2 flies of both sexes, four of the five transcripts (“RA,” “RB,” “RC,” “RD”) were present from larval to adult development whereas “RE” appeared during metamorphosis. “RE” seems to be more abundant in adult males than in females. The 1573-1 mutation drastically affected most transcripts in both sexes: “RA” and “RE” were never detected, whereas the signal corresponding to “RC” largely decreased. On the other hand, “RB” and “RD” showed no apparent change in larvae and adults of both sexes, if compared to both control strains.
Interestingly, “RA” and “RB” transcripts were never detected in D. simulans females and males whereas the pattern of the other three transcripts resembled that of control D. melanogaster: “RC” was apparently more abundant than “RE” and “RD”; “RE” was apparently higher in adult males than in females. The desat2 transcript, which was never detected in D. melanogaster, was present in D. simulans adults of both sexes, with apparently higher levels in males.
Using Q-PCR, we precisely quantified and compared the level of the five desat1 transcripts between 24-hr-old D. melanogaster adults (Table 3). This developmental period was chosen because it is critical for the differentiation of sex pheromones (Wicker and Jallon 1995; Ferveur et al. 1997; Savarit and Ferveur 2002a). “RD” showed the lowest and most constant level in all samples and was chosen as the reference (= 1) to estimate the variation of the four other transcripts. In the two control strains, “RC” was the most abundant transcript and represented 93–97% of the total amount detected, but showed no sexual difference. The quantity of “RC” was drastically reduced in 1573-1 flies and dropped to <1/2000th of its control value. “RA” and “RB” were more abundant in females than in sibling males, whereas “RE,” which was rare, was only slightly more abundant in N2 males. “RA,” which represented 2–5% of the total transcript in the two control strains, was also drastically reduced in 1573-1 flies (22–59 times less abundant than “RD”). On the other hand, “RB,” which dropped to only one-half to one-fourth of its control level, became the predominant transcript (82–87%) in mutant flies. “RE” was never detected in 1573-1 flies. It should be noted that the three most affected transcripts (“RA,” “RC,” and “RE”) are located upstream of the transposon.
TABLE 3.
Comparison of the quantity of five desat1 transcripts in 24-hr-old male and female flies of various genotypes
| Female
|
Male
|
|||||
|---|---|---|---|---|---|---|
| Transcript | Genotype | a.u. | % | a.u. | % | Quantity |
| Canton-S | 275 (41) | 5.58 | 117 (5) | 2.63 | 0.001 | |
| RA | N2 | 232 (27) | 2.83 | 133 (10) | 1.78 | 0.001 |
| 1573 | −22 (5) | 0.17 | −59 (11) | 0.18 | 0.017 | |
| 0.001 | 0.005 | |||||
| Canton-S | 4587 (82) | 93.20 | 4292 (579) | 96.36 | 0.248 | |
| RC | N2 | 8085 (637) | 96.04 | 7289 (1226) | 97.55 | 1 |
| 1573 | 2.4 (0.2) | 8.87 | 0.7 (0.6) | 7.44 | 0.021 | |
| 0.007 | 0.012 | |||||
| Canton-S | 8 (2) | 0.16 | 12 (3) | 0.28 | 0.248 | |
| RE | N2 | 9 (1) | 0.11 | 16 (1) | 0.22 | 0.021 |
| 1573 | 0 (0) | 0 | 0 (0) | 0 | 1 | |
| 0.021 | 0.020 | |||||
| Canton-S | 51 (7) | 1.04 | 32 (4) | 0.71 | 0.043 | |
| RB | N2 | 85 (7) | 1.01 | 33 (1) | 0.44 | 0.021 |
| 1573 | 23 (2) | 87.19 | 7.8 (0.8) | 81.88 | 0.021 | |
| 0.007 | 0.023 | |||||
| Canton-S | 1 | 0.02 | 1 | 0.02 | ||
| RD | N2 | 1 | 0.01 | 1 | 0.01 | |
| 1573 | 1 | 3.77 | 1 | 10.51 | ||
The quantity of each transcript was evaluated in arbitrary units (a.u.) with the amount of “RD” transcript chosen as the reference unit. Data shown correspond to the mean (±SEM) of a duplicated quantification carried out with two independent extractions (N = 2 + 2). Nonparametrical statistics allow us to compare the genotypes (with a Kruskal-Wallis test) and the sexes (with a Mann-Whitney test, except for “RD,” which was constant). The percentage indicates the representativeness of each transcript relative to the total amount of detected transcripts in each sex and genotype. The negative value obtained in 1573-1 flies indicates that the quantity of “RA” was a fraction (1/22 in females, 1/59 in males) of 1 a.u
Characterization of C′1, a desat1 null allele:
Our data suggest that 1573-C′1 is a desat1 null allele. C′1 retained roughly the 5′ part of the transposon with its flanking region, but showed a complete deletion of the 3′ region of the DNA region encompassing all the coding region of desat1. Most C′1/C′1 individuals died during their second larval instar stage (L2), remaining up to 5 days in L2 without being able to moult into L3 (the L2 stage normally lasts 24 hr). We chose to detect and compare the transcriptional pattern of L1 (with both sexes pooled) because the duration of the L1 stage, and the frequency of L1-to-L2 moult were similar in C′1 and control genotypes. With RT-PCR, control L1 showed all transcripts (except “RE”), whereas no transcript was detected in C′1 (Figure 3B). This result suggests that C′1 is a null allele. This hypothesis is supported by the fact that C′1 did not complement the defective CH phenotype induced by mutant and intermediate excision alleles (data not shown).
Four chromosomal deficiencies that theoretically partly or totally uncover the desat1 gene were tested in complementation with the C′1 null allele with regard to viability, CH phenotype, and transcription profile. These experiments yielded varying results: when combined with C′1, the three deficiencies T32, KarH10, and P21, but not ry615, could complement both the developmental viability and the CH phenotype. However, the desat1 transcription pattern that was determined either in adults (for the three former genotypes) or in L1 (for ry615/C′1 that induced lethality between late embryogenesis and L2 stage) was similar to that of the control strains. This indicates that none of these deficiencies completely delete the desat1 gene and are therefore not appropriate to assess whether desat1 is an essential gene required for larval development.
C′1 could also complement adult viability and the CH phenotype of P(w) l(3) S028813, another lethal mutation associated with desat1 (and inducing a mild effect on CHs; Labeur et al. 2002), indicating that both the lethality and the pheromonal defect associated with this allele were perhaps not directly caused by desat1 (data not shown). We did not study the second lethal allele (1573-O7) in detail because it complemented the CH phenotype of all mutant desat1 excision alleles tested (data not shown).
DISCUSSION
Our data indicate that the PGal4 transposon inserted in the regulatory region of desat1 acts recessively to strongly decrease the production of unsaturated CHs of mature Drosophila flies. Precise removal of the transposon rescued a wild-type CH profile. Incomplete excision of the transposon-induced new alleles with a CH phenotype whose gravity was generally related to the size of the fragment inserted in desat1. The desat1 coding region was completely deleted in the C′1 null allele.
Genetic and molecular basis of sexually dimorphic CHs:
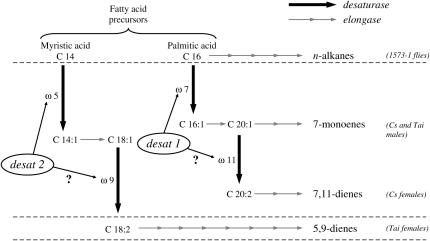
A set of sexually dimorphic characters was highly affected by the mutation. Does the dimorphism of D. melanogaster sex pheromones rely upon only a single desaturase enzyme? Figure 4 shows a simplified representation of the biosynthetic pathway leading to the production of CHs in Cs and Tai females and males together with the possible effect of the 1573-1 mutation in both sexes. With our data, we cannot assess whether the sexual difference for the number of double bonds can be induced by only a single desaturase (coded by desat1) or if the second double bond on carbon ω11, found only in females, is specifically processed by a second (as yet unknown) desaturase. The former hypothesis seems more probable: no CHs with a single double bond on carbon ω11 were detected in our mutant females. Furthermore, Desat1 can process two successive desaturation steps in the silkmoth Bombyx mori (Moto et al. 2004). In this case, desat2, which is active only in Tai-like females, would also create two double bonds successively on ω5 and on ω9 (Figure 4).
Figure 4.
Biosynthetic pathway of pheromones in D. melanogaster flies of different genotypes. The two genes desat1 and desat2 code for two desaturases with different substrate specificity (palmitate and myristate, respectively) that introduce a first double bond on the carbon ω7 (desat1) or on the carbon ω5 (desat2; adapted from Jallon and Wicker-Thomas 2003). Hypothetically, the second double bond (on carbon ω11 for Cs females and on carbon ω9 for Tai females) could be processed by the same Desat enzyme on mono-unsaturated precursors (C20:1 for desat1 and C18:1 for desat2). Subsequently, saturated and unsaturated fatty acid precursors are processed by several elongation steps coupled with a final decarboxylation step (not shown here), leading to the production of mature cuticular hydrocarbons. The desat2 gene, which normally induces the production of 5,9 dienes in Tai-like females, is defective in Cs flies, leading to the predominant production of 7,11 dienes and of 7-monoenes by females and males, respectively. The desat11573-1 mutation largely reduces the levels of these unsaturated hydrocarbons and largely increases the levels of n-alkanes in homozygous mutant flies (1573-1). The solid and the shaded arrows represent the desaturation and the elongation steps, respectively (the number of shaded arrows is not indicative of the number of elongation reactions).
The moderate sexual dimorphism for CHs that persisted between mutant flies was mostly based on the variation of the ratio between n-tricosane (23Lin) and n-pentacosane (25Lin), which were predominant in mutant males and females, respectively. This quantitative difference suggests that a chain-lengthening enzyme, probably an elongase adding two carbons, is more active in mutant females. This situation contrasts with the sexual difference shown by Cs female flies where two elongation steps (2 + 2 carbons) occur together with two desaturation steps that are additively processed on C7 and on C11. Therefore, our data suggest that the activity of Desat1 is coupled with that of an elongase because both enzymes apparently decreased their activity in mutant desat1 females. In Musca domestica, the sex specificity of pheromones depends largely upon the elongation of the carbon chain in male predominant CH (27C; Vaz et al. 1989). The analogous involvement of an elongase on the sexual dimorphism of sex pheromones in the two dipteran species indicates a possible conservation of some of the biosynthetic mechanisms related to pheromonal communication.
Our data reveal two other sex differences: mutant alleles generally induced a higher increase of SumCH in males, whereas intermediate alleles more frequently affected the female's CH profile. We previously hypothesized that desat1 interacts with sex determination genes because the ectopic expression of the dominant feminizing TraF factor, driven by the desat11573-1 PGal4 enhancer-trap line, highly masculinized the predominant CHs of XX flies (Savarit and Ferveur 2002b). It is possible that the overexpression of TraF in the oenocytes and in the fat body affects target genes that interact with desat1. One of the best TraF-dependent candidate genes is doublesex, the manipulation of which affects sex-specific CHs (Waterbury et al. 1999).
Which desat1 transcript(s) could be involved in the sex pheromone difference of control D. melanogaster flies? Among the five detected transcripts that were precisely quantified at the developmental period critical for the differentiation of sex pheromones (24-hr-old adults; Wicker and Jallon 1995; Ferveur et al. 1997; Savarit and Ferveur 2002a), only “RA” and “RB” showed a significant difference between the sexes. “RE” also showed a slight sexual quantitative difference during early adult development, but it is probably not involved because it was completely absent of mutant flies, which still produced a residual amount of sex-specific CHs. Conversely, “RA” and to a lesser extent “RB,” were decreased in mutant flies, but both these transcripts still showed a significant quantitative sexual difference. This indicates that “RA” and/or “RB” could induce the sex specificity of D. melanogaster pheromones. This hypothesis is supported by our findings that neither “RA” nor “RB” was detected in the monomorphic species D. simulans. Nevertheless, our data cannot explain how transcripts could be significantly more abundant in 3-day-old females than in males (Wicker-Thomas et al. 1997), given that “RC,” which is by far the most abundant transcript (>93%) in 24-hr-old adults, showed no sex difference. This discrepancy suggests that “RC” could increase faster in females than in males in the first 3 days of adult life.
Pleiotropic functions of desat1:
Although desat1 and desat2 genes show strong homology in their coding region (Knipple et al. 2002), this does not mean that they have the same function. The fact that desat2 is functional only in Tai-like females, whereas desat1 is functional in all wild-type D. melanogaster flies, suggests that desat1 is an essential gene for this species, whereas desat2 is a variant found in some geographic strains, perhaps linked to a case of incipient speciation (Takahashi et al. 2001; Ting et al. 2001; Fang et al. 2002). The importance of desat1 could be explained by its multiple functions (and transcripts), some of which could be indispensable, unlike desat2 with its simpler regulation and only one predicted transcript.
The desat2 gene has pleiotropic effects and can change reproductive characters and ecologically adaptative features, including resistance to desiccation (Greenberg et al. 2003). Our study suggests that desat1 also has a pleiotropic activity. Apart from the processing of sex pheromones, this gene could also be involved in other aspects of biosynthesis such as changing the overall level of CHs (SumCH), which may be involved in physiological or ecological characters. It is also possible that the desat1 mutation increases the availability of fatty acid precursors or changes the turnover of alkanes that would accumulate faster or remain longer on the fly cuticle than alkenes. The altered desaturase, or some side effects of the transposon, could also interfere with another, as yet unknown, metabolic pathway. Alternatively, the increased SumCH of mutant alleles could indicate that more fatty acid precursors are necessary to process alkenes than alkanes.
We do not know yet whether Desat1 is necessary for larval development. If this is the case, “RC” and/or “RD” should be necessary for larval survival because they were absent in lethal C′1 larvae and present in viable 1573-1 mutants. On the contrary, “RA,” “RB,” and “RE” should not be required for viability because their absence in 1573-1 or in D. simulans flies had no visible consequence on viability. However, a precise chromosomal deficiency that exclusively and completely deletes desat1 needs to be generated to verify that this gene codes for an essential function required for larval development.
Finally, the sexually dimorphic CHs that are altered by the desat11573-1 mutation are secondary sexual characters that are involved in mate choice and can affect the sex ratio of the progeny (Marcillac and Ferveur 2004). This indicates that the desat1 gene could regulate some aspects of the conflict between the sexes (Chapman et al. 1995; Rice 1996). The fact that D. melanogaster female pheromones efficiently prevent interspecific courtship and mating (Coyne et al. 1994; Coyne and Oyama 1995; Savarit et al. 1999) emphasizes the role of desat1 in reinforcing the mechanisms of sexual isolation. By combining molecular, genetic, biochemical, and behavioral approaches, we hope to better understand how desat1 can be related to the evolution of pheromonal communication in Drosophila.
Acknowledgments
We thank E. Logette for help with the Q-PCR, L. Dartevelle and C. Everaerts for technical assistance, and two reviewers for improving the manuscript. This study was supported in part by the Burgundy Region, the Centre National pour la Recherche Scientifique, and the French Ministry of Education and Research.
References
- Antony, C., and J. M. Jallon, 1982. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 28: 873–880. [Google Scholar]
- Bradbury, J. W., and S. L. Vehrencamp, 1998. Principles of Animal Communication. Sinauer Associates, Sunderland, MA.
- Chapman, T., L. F. Liddle, J. M. Kalb, M. F. Wolfner and L. Partridge, 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373: 241–244. [DOI] [PubMed] [Google Scholar]
- Cobb, M., and J. M. Jallon, 1990. Pheromones, mate recognition and courtship stimulation in the Drosophila melanogaster species sub-group. Anim. Behav. 39: 1058–1067. [Google Scholar]
- Cooley, L., R. Kelley and A. Spradling, 1988. Insertional mutagenesis of the Drosophila genome with single P elements. Science 239: 1121–1128. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and R. Oyama, 1995. Localization of pheromonal sexual dimorphism in Drosophila melanogaster and its effect on sexual isolation. Proc. Natl. Acad. Sci. USA 92: 9505–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A., A. P. Crittenden and K. Mah, 1994. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265: 1461–1464. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., C. Wicker-Thomas and J. M. Jallon, 1999. A gene responsible for a cuticular hydrocarbon polymorphism in Drosophila melanogaster. Genet. Res. 73: 189–203. [DOI] [PubMed] [Google Scholar]
- Dallerac, R., C. Labeur, J. M. Jallon, D. C. Knipple, W. L. Roelofs et al., 2000. A delta 9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97: 9449–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak, P., M. M. Omar, R. D. Saunders, M. Pal, O. Komonyi et al., 1997. P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: correlation of physical and cytogenetic maps in chromosomal region 86E–87F. Genetics 147: 1697–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, S., A. Takahashi and C.-I Wu, 2002. A mutation in the promoter of desaturase 2 is correlated with sexual isolation between Drosophila behavioral races. Genetics 162: 781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon, D., A. C. Jung, M. Criqui, B. Lemaitre, S. Uttenweiler-Joseph et al., 1998. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 17: 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur, J. F., 1991. Genetic control of pheromones in Drosophila simulans. I. Ngbo, a locus on the second chromosome. Genetics 128: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur, J. F., and G. Sureau, 1996. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc. R. Soc. Lond. B Biol. Sci. 263: 967–973. [DOI] [PubMed] [Google Scholar]
- Ferveur, J. F., F. Savarit, C. J. O'Kane, G. Sureau, R. J. Greenspan et al., 1997. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science 276: 1555–1558. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido, A., J. M. Del Prado and J. Botas, 1983. The effect of aneuploidy on embryonic development in Drosophila melanogaster. Mol. Genet. 192: 253–263. [Google Scholar]
- Gausz, J., H. Gyurkovics, G. Bencze, A. A. Awad, J. J. Holden et al., 1981. Genetic characterization of the region between 86F1,2 and 87B15 on chromosome 3 of Drosophila melanogaster. Genetics 98: 775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, A. J., J. R. Moran, J. A. Coyne and C. I. Wu, 2003. Ecological adaptation during incipient speciation revealed by precise gene replacement. Science 302: 1754–1757. [DOI] [PubMed] [Google Scholar]
- Jallon, J. M., 1984. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14: 441–478. [DOI] [PubMed] [Google Scholar]
- Jallon, J. M., and J. R. David, 1987. Variations in cuticular hydrocarbons along the eight species of the Drosophila melanogaster subgroup. Evolution 41: 487–502. [DOI] [PubMed] [Google Scholar]
- Jallon, J. M., and J. M. Pechiné, 1989. Une autre race chimique de Drosophila melanogaster en Afrique. C. R. Acad. Sci. Paris Ser. D 309(1): 1551–1556. [Google Scholar]
- Jallon, J. M., and C. Wicker-Thomas, 2003. Genetic studies on pheromone production in Drosophila, pp. 253–280 in Insect Pheromone Biochemistry and Molecular Biology: The Biosynthesis and Detection of Pheromones and Plant Volatiles, edited by G. J. Blomquist and R. G. Vogt. Elsevier Academic Press, Amsterdam.
- Knipple, D. C., C. L. Rosenfield, R. Nielsen, K. M. You and S. E. Jeong, 2002. Evolution of the integral membrane desaturase gene family in moths and flies. Genetics 162: 1737–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeur, C., R. Dallerac and C. Wicker-Thomas, 2002. Involvement of desat1 gene in the control of Drosophila melanogaster pheromone biosynthesis. Genetica 114: 269–274. [DOI] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, London.
- Marcillac, F., and J.-F. Ferveur, 2004. A set of female pheromones affects reproduction before, during and after mating in Drosophila. J. Exp. Biol. 207: 3927–3933. [DOI] [PubMed] [Google Scholar]
- Moto, K., M. G. Suzuki, J. J. Hull, R. Kurata, S. Takahashi et al., 2004. Involvement of a bifunctional fatty-acyl desaturase in the biosynthesis of the silkmoth, Bombyx mori, sex pheromone. Proc. Natl. Acad. Sci. USA 101: 8631–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechiné, J. M., C. Antony and J. M. Jallon, 1988. Precise characterization of cuticular compounds in young Drosophila by mass spectrometry. J. Chem. Ecol. 14 (4): 1071–1085. [DOI] [PubMed] [Google Scholar]
- Pechiné, J. M., F. Perez, C. Antony and J. M. Jallon, 1985. A further characterization of Drosophila cuticular monoenes using a mass spectrometry method to localize double bonds in complex mixtures. Anal. Biochem. 145: 177–182. [DOI] [PubMed] [Google Scholar]
- Reuter, G., J. Gausz, H. Gyurkovics, B. Friede, R. Bang et al., 1987. Modifiers of position-effect variegation in the region from 86C to 88B of the Drosophila melanogaster third chromosome. Mol. Gen. Genet. 210: 429–436. [DOI] [PubMed] [Google Scholar]
- Rice, W. R., 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381: 232–234. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Savarit, F., and J. F. Ferveur, 2002. a Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J. Exp. Biol. 205: 3241–3249. [DOI] [PubMed] [Google Scholar]
- Savarit, F., and J. F. Ferveur, 2002. b Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet. Res. 79: 23–40. [DOI] [PubMed] [Google Scholar]
- Savarit, F., G. Sureau, M. Cobb and J. F. Ferveur, 1999. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Natl. Acad. Sci. USA 96: 9015–9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A., S. C. Tsaur, J. A. Coyne and C.-I Wu, 2001. The nucleotide changes governing cuticular hydrocarbon variation and their evolution in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98: 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting, C. T., A. Takahashi and C. I. Wu, 2001. Incipient speciation by sexual isolation in Drosophila: concurrent evolution at multiple loci. Proc. Natl. Acad. Sci. USA 98: 6709–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz, A. H., E. A. Brownson, G. J. Blomquist and R. C. Reitz, 1989. Sex pheromone biosynthesis in the housefly: evidence for the regulation of the fatty acyl-CoA desaturation and elongation system by 20-hydroxyecdysone. Arch. Insect Biochem. Physiol. 12: 173–186. [Google Scholar]
- Waterbury, J. A., L. L. Jackson and P. Schedl, 1999. Analysis of the doublesex female protein in Drosophila melanogaster: role on sexual differentiation and behavior and dependence on intersex. Genetics 152: 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker, C., and J. M. Jallon, 1995. Hormonal control of sex pheromone biosynthesis in Drosophila melanogaster. J. Insect Physiol. 41(1): 65–70. [Google Scholar]
- Wicker-Thomas, C., C. Henriet and R. Dallerac, 1997. Partial characterization of a fatty acid desaturase gene in Drosophila melanogaster. Insect Biochem. Mol. Biol. 27: 963–972. [DOI] [PubMed] [Google Scholar]
- Wyatt, T. D., 2003. Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge University Press, Cambridge, UK.