Abstract
In Drosophila, the dosage compensation complex (DCC) mediates upregulation of transcription from the single male X chromosome. Despite coating the polytene male X, the DCC pattern looks discontinuous and probably reflects DCC dynamic associations with genes active at a given moment of development in a salivary gland. To test this hypothesis, we compared binding patterns of the DCC and of the elongating form of RNA polymerase II (PolIIo). We found that, unlike PolIIo, the DCC demonstrates a stable banded pattern throughout larval development and escapes binding to a subset of transcriptionally active areas, including developmental puffs. Moreover, these proteins are not completely colocalized at the electron microscopy level. These data combined imply that simple recognition of PolII machinery or of general features of active chromatin is either insufficient or not involved in DCC recruitment to its targets. We propose that DCC-mediated site-specific upregulation of transcription is not the fate of all active X-linked genes in males. Additionally, we found that DCC subunit MLE associates dynamically with developmental and heat-shock-induced puffs and, surprisingly, with those developing within DCC-devoid regions of the male X, thus resembling the PolIIo pattern. These data imply that, independently of other MSL proteins, the RNA-helicase MLE might participate in general transcriptional regulation or RNA processing.
DOSAGE compensation in Drosophila represents a unique example of chromosome-specific upregulation of genetic activity, which results in the equalization of levels of X chromosome products in homo- and hemizygous sexes. This process is shown to be regulated by an RNA-protein complex, called the dosage compensation complex (DCC). The DCC comprises five protein subunits, MSL1, MSL2, MSL3, MLE, and MOF (products of male-specific lethal-1, -2, -3, maleless, and males absent on the first genes, respectively), and at least two noncoding RNAs, roX1 and roX2 (for review, see Meller and Kuroda 2002; Gilfillan et al. 2004). Also there is evidence that a histone phosphokinase JIL-1 participates in the DCC as well as having essential functions in both sexes (Jin et al. 1999; Wang et al. 2001). The dosage compensation in Drosophila is achieved by a twofold increase in transcription from the single male X chromosome. The long-standing model predicts that the acetylase and phosphokinase activities of the DCC specifically modify histones; this renders the chromatin on the male X open, which directly enhances transcription of the X-linked genes (Kuroda et al. 1991; Bone et al. 1994; Jin et al. 1999; Akhtar and Becker 2000; Smith et al. 2000). An alternative model assumes that dosage compensation is caused by an “inverse dosage effect” (Birchler 1996), where DCC functions mainly to sequester the histone modifications and transcriptional factors to the male X, thereby preventing the imbalance of these factors on the X vs. the autosomes that would otherwise lead to an increase in transcription throughout the genome (Bhadra et al. 1999; Pal Bhadra et al. 2005).
On the polytene male X, the DCC is localized in ∼300 discrete regions of decompacted chromatin, referred to as interbands (Kelley et al. 1999). Still, the DCC-binding pattern is far from uniform, since many chromosomal regions were mapped and shown to be reproducibly devoid of DCC binding (DCC gaps) (Baker et al. 1994; Demakova et al. 2003). In general, one of the fundamental questions of the dosage compensation mechanism is what are the factors that determine association of the functional complex with the whole set of its targets, on the one hand, and arrest it in distinct regions on the other hand. Of hundreds of DCC-binding sites along the male X chromosome ∼70 associate with an incomplete complex (Lyman et al. 1997; Gu et al. 1998; Demakova et al. 2003). These were proposed to be DNA targets serving as chromatin entry sites (CESs) and playing a key role both in recruitment of DCC to the X chromosome and in subsequent complex spreading to all additional sites (Lyman et al. 1997; Kelley et al. 1999; Park et al. 2002). Further studies on DCC binding to the X under varying levels of MSL2 have led to the idea that this process is directed by a hierarchy of target sites displaying different affinities for the complex (Demakova et al. 2003). Nevertheless, this model accepts that, finally, upon achievement of high DCC titers, the CES might provoke complex spreading into adjacent low-affinity sites. Unfortunately, it still remains unknown what CESs are in molecular terms. Moreover, some authors suggest that CESs do not possess any special molecular properties except their high affinity to the complex (Fagegaltier and Baker 2004).
It was shown earlier that roX RNAs, as well as histone acetyltransferase and ATPase activities of MOF and MLE, respectively, are needed for recruiting the DCC to multiple non-CES binding sites (Gu et al. 2000; Park et al. 2002). Recently, it was reported that the DCC-binding pattern on the male X reflects the distribution of genes, which are active in the tissue at this moment of development (Sass et al. 2003). Thus, the transcriptional activity of a locus could be determinative for DCC binding to most of its targets. The same idea has also been used to explain discontinuous DCC spreading patterns from autosomal roX transgenes (Kelley et al. 1999; Sass et al. 2003).
To test this hypothesis and to further understand the requirements of additional non-CESs for DCC targeting, we compared the binding patterns of DCC and of PolIIo, the form of RNA polymerase II engaged in efficient transcription (Weeks et al. 1993; Komarnitsky et al. 2000; Cheng and Sharp 2003). Productive transcription is characterized by the phosphorylation of the carboxy-terminal domain (CTD) at serine 5 and 2 in the largest subunit of PolII and the recruitment of a number of elongation and RNA-processing factors (Hampsey and Reinberg 2003; Palancade and Bensaude 2003). In this study, we make use of antibody H14, which recognizes PolII molecules carrying the phosphoserine 5 epitope. Since phosphorylation of serine 5 is required to initiate elongation complex assembly (Komarnitsky et al. 2000; Palancade and Bensaude 2003) and is found in vivo distributed across the entire transcription unit (Schwartz et al. 2003), the H14 staining patterns mark entire active areas along the polytene male X.
We tracked visible changes of PolIIo and DCC distribution through larval development both on the male X and on an autosomal region with DCC spreading from a roX1 transgene. Temporal changes in the expression of distinct genes active in the salivary gland are accompanied by puffing of distinct chromosome regions in well-described patterns that mark the steps of development through larval and pupal stages (Ashburner et al. 1974; reviewed in Zhimulev 1999). Therefore, we were able to compare binding patterns of the proteins with the main visible developmental changes of gene expression.
We found that transcriptionally active areas can be revealed within all the DCC gaps, and in some cases these corresponded to the ecdysone-induced puffs. While the PolIIo appeared dynamic and predominantly associated with puffs, DCC was reproducibly absent from them and generally demonstrated surprising stability of binding patterns on the male X during larval developmental stages. Moreover, in the regions where both proteins are abundant, complete colocalization at the electron microscopy (EM) level appears not to be a rule. We also found that in the ectopic DCC spreading system, similarly to the wild-type male X, a number of transcriptionally active regions are reproducibly devoid of DCC binding. These data combined indicate that many active genes escape DCC binding, suggesting that the transcriptional activity could be necessary but not sufficient for DCC recruitment and stabilization at all its targets.
In this study, we also tested the expectation that, in addition to its proposed function in assembling roX RNAs into DCC (Meller et al. 2000), the RNA-helicase MLE might direct the complex to active genes by associating with nascent transcripts (Richter et al. 1996; Stuckenholz et al. 1999). Since both the data on its localization on polytene chromosomes and the interpretations of these data are still contradictory (Kuroda et al. 1991; Lee et al. 1997; Bhadra et al. 1999; Ruiz et al. 2000), we set out to map all MLE targets in the nucleus. To this end, we separately investigated the distribution through larval development of MLE in both sexes in a range of wild-type and mutant backgrounds as well as upon heat-shock treatment. Our data indicate that, separately from the DCC, MLE associates dynamically with all puffs, thus resembling the PolIIo distribution. This type of puff stage-dependent binding is demonstrated by MLE on female X chromosomes, on autosomes of both sexes, and, surprisingly, in puffs developing within some DCC gaps on the male X chromosome—that is to say, whenever it functions independently of the other MSL proteins. Thus, it is tempting to assume that MLE might contribute to the general mechanisms of transcription regulation and RNA processing in addition to its still-unknown functions in dosage compensation.
MATERIALS AND METHODS
Fly strains and genetic crosses:
Flies were raised on standard cornmeal-yeast-agar-molasses medium. Descriptions of all mutants and rearrangements not specifically mentioned can be found in Lindsley and Zimm (1992). To analyze MLE binding to the X chromosome, the stock w; msl3 [w+; H83M2-61]/TM6, Tb carrying the MSL2-expressing transgene was used (Kelley et al. 1995). The stocks w; msl1L60/CyO and msl3P red/TM6, Tb bear null alleles for msl1 and msl3, respectively (Gorman et al. 1995; Chang and Kuroda 1998).
To generate males with extensive DCC spreading from the autosomal roX transgene, we utilized two stocks: y w roX1− roX2− cos4D; CyO, GM roX1/+ (Park et al. 2002) and y w roX1ex6; GM roX1-ΔDHS-72D (Bai et al. 2004). The females of genotype y w roX1− roX2− cos4D; +/+ were crossed to the males from the second stock. The sons of the genotype y w roX1− roX2− cos4D; GM roX1-ΔDHS-72D were used for cytological analysis.
All crosses to generate larvae for immunostaining were carried out at 18°. A laboratory stock of Drosophila simulans was used.
Staging of larvae:
Each developmental stage of third instar larvae or prepupae displays a specific and exclusively constant puffing pattern. To classify a puff stage (PS) correctly, we used a detailed schedule of puff changes in ontogenesis, which was established earlier (reviewed in Zhimulev 1999) and is routinely used for this purpose.
Immunofluorescent staining:
The immunostaining procedure was as in Kuroda et al. (1991) and as in Demakova et al. 2003). Both primary affinity-purified rabbit anti-MSL2 and anti-MLE antibodies were used at a dilution of 1:100, and anti-MSL1 was used at 1:50 and detected with a 1:150 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG secondary antibodies (Sigma, St. Louis). Primary affinity-purified goat anti-MLE antibodies were used at a 1:150 dilution and detected with a 1:400 dilution of FITC-conjugated rabbit anti-goat IgG secondary antibodies (Sigma). Primary affinity-purified goat anti-MSL3 antibodies were used at a 1:50 dilution and detected with a 1:500 dilution of Cy3-conjugated donkey anti-goat IgG secondary antibody (Rockland, Gilbertsville, PA). Primary monoclonal H14 mouse antibodies against the CTD of RNA polymerase II phosphorylated at Ser5 (Covance) were used at a dilution of 1:50 and detected with a 1:250 dilution of FITC-conjugated goat anti-mouse IgM secondary antibody (Sigma). For double-staining experiments, the antibodies raised in different hosts were incubated simultaneously overnight at 4° in a humidified chamber. After primary antibody incubation, slides were thoroughly washed in PBT and subsequently incubated with the secondary antibodies, first specific for one antigene and then specific for the other (2 hr each). Chromosomes were viewed using epifluorescent optics with the Olympus microscope (Japan) or with Axioscope 2 plus (Zeiss). Images were obtained and treated using the corresponding software: DPController 1.2.1.108 for Olympus and ISIS–CPD1E for Axioscope.
The descriptions of protein-binding patterns are based on the data obtained from at least five slides (∼100–120 nuclei in total) in each experiment.
Heat-shock treatment:
To obtain heat-shocked salivary glands, third instar larvae were collected in a polypropylene tube and submerged in a 37° water bath for 30 min. Squashes were made and immunostained as described above.
Electron microscopy double immunostaining:
For these experiments the transgenic w; msl3 [w+; H83M2-61]/TM6, Tb females were used, since their two X chromosomes in most of the regions demonstrate the DCC pattern resembling that of the wild-type males (Demakova et al. 2003) but display the morphology, which is more convenient for electron microscopy analysis (Semeshin et al. 2002). The immunostaining procedure was as in Semeshin et al. (2002) with minor modifications. Primary affinity-purified rabbit anti-MSL2 antibodies and primary monoclonal H14 mouse IgM antibodies against the phosphorylated at Ser5 CTD of RNA polymerase II (Covance) were used at a dilution of 1:50. For EM colocalization experiments, the antibodies were incubated simultaneously overnight at 4° in a humidified chamber. Then slides were washed four times for 5 min in PBT and then incubated at room temperature for 3 hr with a mixture of secondary antibodies: anti-mouse IgM FITC-conjugated (developed in goat) (1:250) and anti-rabbit IgG Gold (6 nm) conjugated (developed in donkey) (1:30) (Jackson ImmunoResearch, West Grove, PA) antibodies. After incubation with secondary antibodies, the slides were washed with double-distilled water not fewer than six times (3 min each), and then chromosomes were treated with Silver Enhancement reagent (Boehringer Mannheim, Indianapolis) for 20 min to increase the size of gold particles. Thereafter, slides were washed six times (3 min each) with double-distilled water and incubated with anti-goat IgG Gold (18 nm) conjugated antibodies (developed in donkey) (1:30) (Jackson ImmunoResearch) for 3 hr at room temperature, thoroughly washed in PBT and double-distilled water, dehydrated in a graded ethanol series (20, 35, 50, and 70%) for 5 min in each, and left overnight in a 1.5% solution of uranyl acetate in 70% ethanol for staining. Further procedures of dehydration and embedding in epoxy resin have been described elsewhere (Semeshin et al. 1998). Ultra-thin sections were examined in a JEM-100C electron microscope at 80 kV.
To localize chromosome regions, we referred to the revised cytological maps of polytene chromosomes of C. B. Bridges (represented in Lindsley and Zimm 1992).
RESULTS
DCC binding does not accumulate at a subset of transcriptionally active regions, including highly expressed X-linked genes during larval development:
The reason for the reproducible lack of DCC binding in at least 30 regions, including up to several interbands (Demakova et al. 2003), is unclear. Taking into account the recent results arguing in favor of the direct correlation of the DCC-binding pattern with active genes on the X (Sass et al. 2003), one reasonable expectation might be that these DCC gaps are transcriptionally inactive. Having compared the X chromosome binding patterns of the MSL3 protein and of PolIIo in third instar larvae from PS1-2 to PS11, we found that (i) the localization of DCC-negative regions remains unchanged during this time and (ii) RNA polymerase binding sites map within all the visible DCC gaps (Figure 1 demonstrates data on the distal half of the X chromosome). Control tests revealed that the staining sites are dependent on and specific to the primary antibody used and that there is no cross-reactivity of secondary antibodies (data not shown). Thus, DCC-negative regions do contain transcriptionally active genes.
Figure 1.
The distribution of DCC and PolIIo in the distal half of the male X chromosome. The wild-type male X chromosome is stained by anti-MSL3 antibody (red) and anti-PolIIo (green). (A and C) From top to bottom: merged image, DCC, RNA polymerase II, and phase contrast. Asterisks indicate DCC gaps, where distinct RNA polymerase binding sites are seen (green arrows). Merged images demonstrate examples in which MSL3 is detected at sites where RNA polymerase II is absent (separate red arrows), along with the cases in which the signals lie close to each other or partially overlap (adjacent arrows). (B) Fluorescence intensity profile for the two antibodies through the divisions 1–6 of the polytene chromosome map. Bar, 5 μm. This image was obtained on Axioscope 2 plus (Zeiss) and processed with the ISIS–CPD1E software (http://www.metasystems.de).
Some DCC gaps provide morphological evidence for the localization of transcriptionally active areas within them. One of the most prominent DCC gaps maps to the polytene region 3C 7-11. This region harbors a well-known cluster of genes coding for the salivary gland glue components: Sgs-4, Pig-1, ng-1, ng-2, ng-3, and ng-4. Their expression from PS1-2 to ∼PS5 (Furia et al. 1993) manifests as a puff. While the DCC is reproducibly absent from this region during the whole third instar, the PolIIo pattern appears dynamic and varies from intensive diffuse labeling of the puff material at PS1-2 to two weak sites at PS11 (Figure 2, A–D). It should be noted that Sgs4 is known to be dosage compensated (Breen and Lucchesi 1986; Kaiser et al. 1986; Chiang and Kurnit 2003). In addition to the prominent puffs, there are several dozens of so-called “small puffs” on the X chromosome, which do not form any significant swelling of the polytene chromosome in the corresponding region (Belyaeva et al. 1974). For example, such a small puff is described to form in the region 5A1 at PS11, where the two adjacent bands split. PolIIo diffusely paints the puff material; however, no specific MSL binding sites are observed in this region throughout the larval stages, and a typical DCC gap is formed (Figure 2, E–I). A number of other regions on the X chromosome that undergo puff formation during the third instar stage, such as 2C1-2 (Figure 3A), 9CD, and 16B (data not shown), also appear completely devoid of DCC.
Figure 2.
The distribution of DCC and PolIIo within the puffs developed in the distal part of the male X chromosome. The distribution of PolIIo, but not of MSL3, changes within the region 3A-D as a function of larval development (A–D). (A and D) Anti-MSL3 detection. (B and C) Merge of MSL3 (red) and PolIIo (green). A strong RNA polymerase II site is observed at PS1-2 in puff 3C8-12 (B). At PS11, only two faint sites can be seen in this region (C). Borders of the DCC gap in 3C7-11 remain unchanged (marked with white bars). Arrows indicate the sites in the 3AB region where PolIIo is detectable at a given PS (solid arrowheads) and undetectable at a different PS (open arrowheads). The small puff in region 5A developed at late PS11 is bound by PolIIo, but not by MSL3 (E–I). (E and H) Phase contrast. (F and G) Anti-MSL2 detection. (I) Merge of MSL3 (red) and PolIIo (green). Two neighboring bands can be seen in region 5A at PS1-2 (E and F); the interband in between shows no staining for MSL2. At PS11 this region forms a small puff at 5A1 (G and H), which shows intensive binding of RNA polymerase II (I); however, no novel binding sites for MSL2 (G) and MSL3 (I) are observed. PolIIo, but not MSL3, disappears from the 2B3-5 region at late PS11 due to Broad-Complex inactivity (J). From top to bottom: anti-MSL3 detection, anti-PolIIo detection, merge of MSL3 (red) and PolIIo (green). With Broad-Complex becoming inactive during PS10-11, the PolIIo gap is detectable in the 2B3-5 region at this developmental stage (the X chromosome fragment is well stretched). In contrast, MSL3 remains bound to the 2B3-5 region. Bar, 5 μm.
Figure 3.
Localization of DCC and MLE looks different in the regions of prominent puffs developed in the distal part of the X chromosome. (A–D) Oregon-R (wild type) male. (E) w; msl3 [w+; H83M2-61]/TM6 transgenic female. (F) Oregon-R female. (A and C) Anti-MSL2 detection. (B, D, E, and F) Anti-MLE detection. In the distal part of the X chromosome, temporal changes of gene expression are manifested by dynamic puffing in the regions 3C8-12 (from PS1 to PS4), 2B3-5 (from PS2 to PS10), and 2C1-2 (from PS5 to PS8). DCC associates differentially with the prominent 2B puff and is absent from the developed 2C (A) and 3C (C) puffs, whereas MLE binds diffusely to all these puffs (B and D). MLE binding appears intensive in the 3C puff in transgenic females, which produce less DCC than the wild-type male does (E). MLE predominantly binds puffs on female X chromosomes (F). Bar, 5 μm.
However, there is an important exception to these observations, namely the largest X chromosomal puff in the region 2B. Its formation throughout the larval and prepupal stages is mainly due to the activity of the ecdysone-dependent gene Broad-Complex, which maps to region 2B3-5 (Zhimulev et al. 1995). DCC localizes to this puff during all the larval developmental stages. However, in contrast to the diffuse PolIIo labeling of all the puffs, the DCC pattern in the region 2B appears as distinct differential binding sites lying across the puff material even during maximal puff development (Figure 3A). As this region has recently been demonstrated to harbor over five CESs in msl3 mutants (Demakova et al. 2003), it is tempting to suggest the MSL binding in 2B is mainly due to the molecular peculiarities of local CESs, rather than to the transcriptional activity of the underlying genes. In accordance with this idea, we found that PolIIo escapes from the region 2B3-5 at PS10-11 during the short period when the gene Broad-Complex is known to be inactive (Andres et al. 1993), whereas MSL3 binding remains unchanged (Figure 2J).
In general, in contrast to the PolIIo distribution, the DCC pattern looks stable over the developmental stages analyzed (region 3A-D in Figure 2, B and C). Some of the faint DCC-binding sites bordering some gaps, as exemplified by the regions 3C7-11 or 6EF, may appear undetectable in some nuclei of a single individual.
Discontinuous spreading of the DCC from a roX1 transgene does not fully reflect localization of active genes in the flanking autosomal region:
We asked whether DCC chooses active genes when it spreads locally from autosomal roX transgenes. To generate the most efficient DCC spreading in all nuclei, we tested male larvae, which carry the GM roX1-ΔDHS-72D transgene in a roX1−roX2− double-mutant background (Park et al. 2002; Bai et al. 2004). We found that a large number of good Po1IIo-binding sites, including some of ecdysone-induced puffs, do not associate with the DCC (Figure 4, A and C). The DCC spreading patterns are well known to be variable from nucleus to nucleus, although we failed to confirm this variability to be PS dependent (Figure 4, B and D). It should be noted that in the region 63BC the DCC binds one site, which was found to associate often with the complex in wild-type males (Demakova et al. 2003) and never binds to neighboring sites regardless of their active transcriptional status (Figure 4, A–D). Thus, similarly to the male X, the patterns of ectopic DCC binding do not directly reflect the localization of active transcriptional domains in autosomes carrying a GM roX1-ΔDHS-72D transgene.
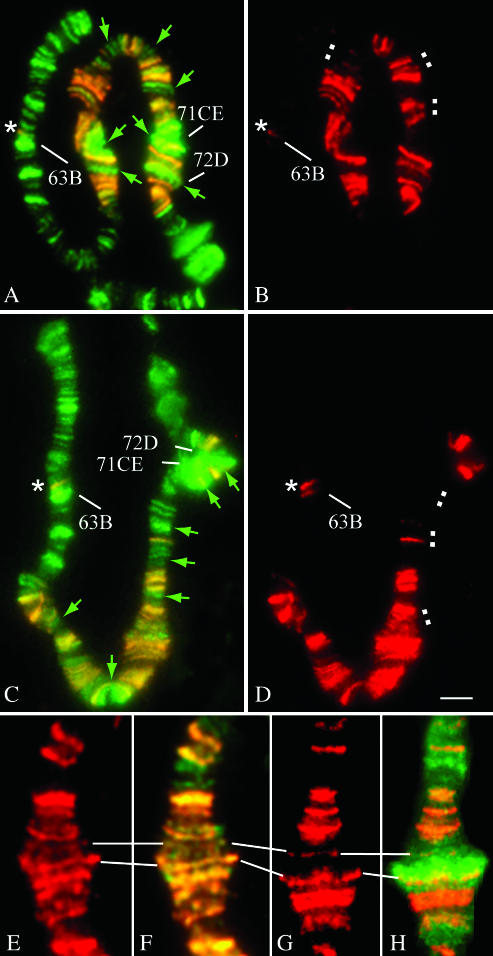
Figure 4.
The distribution of DCC and PolIIo within the region 66B-72D of chromosome 3 carrying a roX1 transgene. (A–D) The distribution of DCC and PolIIo within the 66B-72B region in two nuclei from a single salivary gland (PS5). (A and C) Merge of MSL3 (red) and PolIIo (green). (B and D) Anti-MSL3 detection. Green arrows indicate DCC gaps, which contain PolIIo-binding sites, including some of the ecdysone-induced puffs (A and C). White squares indicate distinct sites, where DCC binding demonstrates variability (B and D). (E and H) The distribution of DCC and PolIIo within the chromosomal region 68C. (E and F) PS5. (G and H) PS3. At PS3, the development of a large puff is associated with a novel strong PolIIo-binding site while the DCC pattern looks unchanged except for a novel DCC gap in the region 68C. An asterisk indicates the chromosomal region 63BC where the DCC skips over the site of high transcriptional activity, but reproducibly binds to another site in 63B, which often associates PS independently with the DCC in wild-type males (Demakova et al. 2003). Bar, 5 μm.
EM double immunostaining reveals that DCC and Po1IIo can map to close but still distinct areas within a single interband:
Since the majority of factors involved in transcription localize on polytene chromosomes to the regions of decondensed chromatin, namely to interbands and puffs (Champlin et al. 1991; Weeks et al. 1993; Stokes et al. 1996; Kaplan et al. 2000; Gerber et al. 2001; Gonzy et al. 2002; Saunders et al. 2003), it is no wonder that light-microscopy analysis of the DCC and PolIIo patterns on the male X revealed a significant degree of overlap in many regions (except puffs and cytologically extensive DCC gaps). However, we noted a number of regions where distinct MSL-binding sites could be observed, whereas the RNA polymerase was undetectable (Figure 1). These might be classified as some of the CESs since unknown DNA features within CESs may serve as targets for the MSL proteins. Additionally, the recently characterized CES within the 18D region appears not to be transcribed (Oh et al. 2004). Nevertheless, many of such regions seem not to correspond to the known 70 CESs.
Upon closer examination of the well-stretched chromosomes, we managed to reveal also a number of chromosomal regions, in which the signals tend to show only partial overlap or to border each other (Figures 1 and 2, A–D). To determine precisely the relative localization of DCC and PolIIo within distinct small chromosomal regions, we carried out EM double immunostaining. Figure 5 shows data obtained for the 6A-D and the 8E-10A regions. Earlier it was shown that MSL2 tends to demonstrate unipolar labeling of narrow band/interband borders along the whole male X (Semeshin et al. 2002). Here we found that this specific localization of MSL2 looks different from PolIIo binding to decompacted interbands (Figure 5, C–F). The protein labels look partly overlapping in some regions (Figure 5, D–F) but localize apart in others (Figure 5C). Thus, EM data also indicate that relative localization of PolIIo and MSL2 within a distinct interband may be very close but still not identical.
Figure 5.
Detailed relative localization of MSL2 and PolIIo within distinct chromosomal regions at the electron microscopy level. The w; msl3 [w+; H83M2-61]/TM6, Tb female X chromosomes are double labeled with PolIIo-specific (small grains) and MSL2-specific (large grains) antibodies. (A and B) The regions 6A-D and 8E-10A, respectively. Bar, 1 μm. (C–F) Magnified insets (outlined by white rectangles) shown in A and B: (C) PolIIo grains cover decompacted chromosomal material between the bands 6C6-7 and 6D1-2 with a prominent MSL2-binding site marking the distal border of the band 6C6-7. The proteins appear to map to neighboring although distinct areas. (D) MSL2 localizes to the proximal border of band 8E1-4, whereas PolIIo maps immediately adjacent, in a decompacted area between the bands 8E1-4 and 8E7-8. MSL2 and PolIIo look partly colocalized. (E) MSL2 demonstrates intensive binding to the band 9C3-4, while PolIIo grains occupy the neighboring decompacted area between 9C3-4 and 9D1-2. We consider this as an example of a partial overlap. (F) PolIIo and MSL2 grains look colocalized at both sides of the band 9E3-4. In contrast, PolIIo appears not to coincide with the location of MSL2 in the neighboring region, in which MSL2 decorates the border of the band 9E7-8 while PolIIo maps somewhat distally to the decompacted body of the interband. Bar, 1 μm.
MLE protein interacts with numerous transcriptionally active regions of polytene chromosomes independently of DCC:
MLE possesses NTPase and both RNA- and DNA-helicase activities (Kuroda et al. 1991; Lee et al. 1997). Furthermore, the physical binding of MLE to the X chromosome most likely occurs via RNA (Richter et al. 1996). It is generally believed that MLE may act as a helicase to facilitate the targeting of the MSL proteins to male X or assembly of DCC (Pannuti and Lucchesi 2000). Nevertheless, the possibility that the RNA-helicase activity of MLE could play a significant role in DCC stabilization in the regions of active genes via interaction with nascent transcripts has not been excluded to date (Richter et al. 1996; Stuckenholz et al. 1999). In contrast to other MSLs, MLE is expressed in both sexes in comparable quantities (Kuroda et al. 1991) and can contribute to processes other than dosage compensation (Kernan et al. 1991; Rastelli and Kuroda 1998; Reenan et al. 2000).
Although MLE is the first DCC subunit that has been immunolocalized on the polytene chromosomes, it is the only one of the MSLs whose detailed localization still remains unclear. MLE was first reported to bind predominantly the male X chromosome and additionally to demonstrate numerous faint sites of association on male autosomes and all female chromosomes, these autosomal targets being uncharacterized (Kuroda et al. 1991). Later, Bhadra et al. (1999), using the same MLE-specific antibody, restricted male MLE binding exclusively to the X chromosome. At the same time, in females, they reported msl-dependent MLE binding to all chromosomes, and this finding catalyzed an idea about the existence of a reduced MSL complex in females that might contribute to an inverse dosage effect (Bhadra et al. 1999). Finally, recently it was shown that in addition to labeling the X chromosome, MLE binds only a few autosomal sites in males, similar to other MSLs (Ruiz et al. 2000).
To gain further insight into possible roles of MLE in transcription regulation, we performed precise mapping of MLE-binding sites in both sexes at different points of larval development. First, we noted that MLE autosomal distribution looked identical in both sexes. As demonstrated in Figure 6, A and B, the RNA-helicase MLE binds essentially to all the developmental puffs typical for the PS analyzed and to many interbands. The observation that MLE binds puffs also is true for the female X chromosome (Figures 3F and 6B). Diffuse PS-dependent binding sites on female X look quite different when compared with the reproducible banded pattern in males. Also, we found that several regions (18F, 19A, 22B, two sites in 29C, 30A, 50C, and 86C) reproducibly demonstrate intensive MLE staining, some of them appearing PS specific (Figure 6, A and B). To illustrate, the strong MLE-binding site in the region 30A can be detected only at PS1, whereas the one in the region 29C appears exclusively at the PS8-9. Thus, when associating with the DCC, the MLE distribution pattern on the male X is highly reproducible, whereas its MSL-independent chromosomal binding appears to be essentially a function of the transcriptional status of the region, resembling the PolIIo pattern. Additionally, we observed punctate MLE staining of the chromocenter with the intensity of staining varying from nucleus to nucleus (Figure 6, A, B, and D), as it was shown earlier for the species from the Obscura group (Bone and Kuroda 1996). Under the staining conditions used, no MLE-binding sites could be detected in mle1/mle1 females (data not shown).
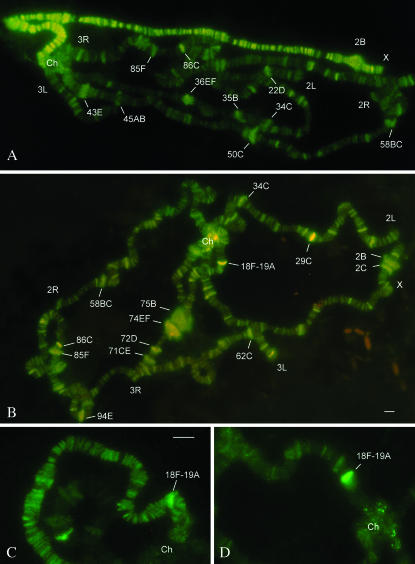
Figure 6.
MLE localization on salivary gland polytene chromosomes. (A) Oregon-R male. (B) Oregon-R female. MLE predominantly binds to major developmental puffs and also to many interbands. Some binding sites look very strong, for example, those in the 18F-19A region, which are detectable both on male (C) and on female (D) X chromosomes. Bar, 5 μm.
It is of special interest to understand whether MLE is able to function independently from DCC on the male X. As an intriguing exception, we detected MLE in puffs developed in the regions 2C, 3C, 16AB, that is, in the DCC gaps. (Figure 3, B and D). With the Sgs4 cluster becoming inactive, the prominent MLE-binding site in the region 3C appears to regress, but at PS5-6 we still were able to detect one faint signal additionally to the DCC pattern typical for the region (Figure 3B). The MLE labeling of the Sgs4 puff is observed also in w; msl3 [w+; H83M2-61]/TM6, Tb female larvae (Figure 3E). These animals carried the MSL2-expressing transgene and therefore exhibited ectopic dosage compensation (Kelley et al. 1995) but produced the DCC at a lower level compared to that of the wild-type male. We speculate that reduced amounts of DCC might be the reason for much more intensive puff labeling by anti-MLE antibodies in the transgenic females. This proposal implies that there might be a competition for MLE between DCC and DCC-independent target sites on the male X (first of all, in the regions devoid of DCC). This might explain why MLE binding in the chromocenter appears to be more detectable in female cells than in male ones, where most of the protein is recruited to the X chromosome. Also, we observed, first, that unlike DCC, MLE demonstrates diffuse labeling in the 2B puff (Figure 3B) and, second, that the strongest MLE-binding sites sometimes can be seen in the 18F-19A region (Figure 6, C and D). We found these sites to be typical for females but never to serve as strong targets for the other MSL proteins. Thus, we believe that the RNA-helicase MLE has an additional function, separate from its participation as a DCC subunit on the male X.
MLE associates with heat-shock-induced puffs and apparently shows increased affinity to the αγ-element, which produces the noncoding heat-shock RNA:
Upon brief exposure to heat shock, the MLE protein can be found in many heat-shock-induced puffs. In particular, we observed a very robust permanent signal in the region 87C. Strong labeling could also be seen in the puff regions 93D and 95D, but never in the region 87A (Figure 7, A and B). The two adjacent puffs 87A and 87C are known to develop due to the activity of a cluster of hsp70 genes (Ish-Horowicz et al. 1979). However, in contrast to the 87A region, the 87C region contains not only the hsp70 cluster, but also the αγ-element, coding for the hshRNA with unknown functions (Sharma and Lakhotia 1995). Since we never observed any MLE-binding site in region 87C in both sexes under non-heat-shock conditions, we speculate that this particular hshRNA, which belongs to the same class of RNA molecules as the roX RNAs, might be the target for MLE. To test the idea, we utilized D. simulans, a sibling species for D. melanogaster, which lacks the sequences homologous to the αγ-element in this region (Lis et al. 1981). Consistent with this proposal, when staining the polytene chromosomes from D. simulans heat-shocked larvae with MLE-specific antibodies, we failed to detect labeling of the 87C region (Figure 7C). It should be noted that αγ-element RNA sequence, when compared to that of the roX1 or roX2, produced no significant homologies (http://www.ncbi.nlm.nih.gov/blast/).
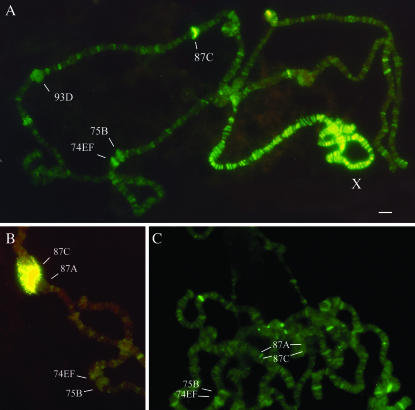
Figure 7.
MLE associates with a number of heat-shock-induced puffs. (A) Polytene chromosomes of the D. melanogaster male following 15 min of heat shock at 37°. MLE appears in the developing heat-shock genes; the strong signal in the 87C region is shown. (B) Well-developed heat-shock puffs in the 87A and 87C regions. A prominent MLE-binding site is observed in only one of the puffs. (C) No significant MLE staining in the 87C region on unpaired homologs of chromosome 3R of D. simulans. Bar, 5 μm.
Finally, by using the female larvae from the stocks w; msl1L60/CyO and msl3P red/TM6, Tb, which were homozygous for the msl1 and msl3 null alleles, respectively, we ascertained that neither of these mutations caused any detectable changes in MLE0binding patterns on the chromosomes (data not shown). This result is inconsistent with the idea that MLE association with female chromosomes is MSL dependent as described by Bhadra et al. (1999).
Thus, independently of other MSL proteins, MLE interacts with the most prominent transcriptionally active regions of chromosomes and likely shows increased affinity to the transcripts of certain genes.
DISCUSSION
The nature of targets for the DCC is a long-standing problem. Originally, it was proposed that specific enhancer-like sequences might reside close to individual X-linked genes, serving as targets for DCC (reviewed in Baker et al. 1994). In contrast, the “spreading” model postulates that the male X is marked by a quite limited number of DNA sequences (∼35) that recruit the DCC and accumulate locally at high levels, which in turn results in association of the complex with numerous sites of low affinity (Kelley et al. 1999; Kageyama et al. 2001). Nevertheless, the modern view of the problem assumes that there might be many more DNA sequences required both for the initial recruitment/assembly of DCC (CES) (Demakova et al. 2003) and for the association of a functional complex with additional sites (non-CES) (Fagegaltier and Baker 2004; Gilfillan et al. 2004; Oh et al. 2004). In contrast to these postulated DNA sequences, most sites on the X, which are targets for functional DCC, are thought to mark genes actively transcribed in a given tissue and time of development (Kelley et al. 1999; Sass et al. 2003). This idea implies that DCC mediates transcription enhancement via direct involvement in transcription regulation of each active gene. In this article, we made an effort to test further this model by precisely investigating the relative localization of DCC and PolIIo along the male X in the course of larval development.
Previously it was described that in vivo PolIIo and various elongation factors, as well as the H3.3 histone variant, dynamically associate with active genes, accompany their expression, and look colocalized in Drosophila polytene chromosomes. This overlap is most obvious as diffuse labeling of developmental and heat-shock-induced puffs (Weeks et al. 1993; Kaplan et al. 2000; Gerber et al. 2001; Saunders et al. 2003; Schwartz et al. 2003; Schwartz and Ahmad 2005). Intriguingly, we found that DCC demonstrates striking stability in both the number and intensity of binding sites along the X throughout larval development. Moreover, despite being targets for MLE, the sites of the most intensive gene expression both on the male X and within an autosomal DCC-spreading area appear to not be targets for DCC at all. We also demonstrated all the DCC gaps that comprise active genes. Additionally, DCC skips over a number of transcriptionally active regions when it inappropriately spreads in cis from an autosomal roX1 transgene. We therefore suggest that active transcriptional status of the chromosomal region or association with MLE is not sufficient for DCC targeting and that, if DCC binds to actively transcribed regions, it does so very selectively.
Our findings on MLE localization on the polytene chromosomes might reflect dual functioning of MLE on the male X. In addition to being a subunit of DCC, MLE probably accomplishes some unrelated functions, which are neither X nor sex specific. On the basis of the observed MLE association with a large number of sites of active transcription in both sexes, we believe that this RNA-helicase might be important not only for splicing certain genes, as was shown for the gene para (Reenan et al. 2000), but also for playing some general role in the transcription process. In support of this idea, the mammalian MLE homolog, RHA, was demonstrated to contribute to various steps of transcription—from initiation to processing of nascent transcripts (Zhang and Grosse 2004). Assuming that MLE apparently is able to bridge DCC with transcriptionally active regions, this might occur only if some additional requirements for DCC binding are realized.
Earlier, it was reported that a partial MSL complex lacking MSL2 protein is present in normal female nuclei. Accordingly, mutations in various msl genes except mof disassociate all MSLs from the chromosomes in females (Bhadra et al. 1999). Nevertheless, our data on MLE distribution in polytene chromosomes of females homozygous for msl1 or msl3 null alleles indicate that even if MSLs form a partial complex in females, MLE binds the chromatin in an MSL-independent manner.
Our findings raise questions as to what are the reasons for exclusion of DCC from some active X-linked regions and whether dosage compensation does take place there. One can speculate that highly active chromatin in puffing regions turns into a poor substrate for the DCC due to drastic changes in packaging, possibly, up to nucleosome removal (Orphanides and Reinberg 2000; Kireeva et al. 2002). However, a cluster of CESs bound by DCC is detected in the puffed 2B region throughout larval development. Moreover, strong transcription induced in EP transposons on the male X sometimes results in ectopic DCC recruitment, suggesting that the complex is able to recognize very active chromatin (Sass et al. 2003).
Revealing active genes within each of the cytologically extensive DCC gaps provides yet another puzzle. On the one hand, in the neo X chromosome of D. miranda, the blocks of chromatin escaping dosage compensation do alternate with other blocks that are dosage compensated and therefore bind DCC (Bone and Kuroda 1996; Marin and Baker 1998). However, no data indicate that such clustering takes place in D. melanogaster (Ghosh et al. 1992). If X-linked genes actually possess still unknown features needed for DCC targeting (Fagegaltier and Baker 2004), then DCC gaps might reflect evolutionary incompleteness of this process in D. melanogaster. It should be noted that, in contrast, ∼70 autosomal regions are competent to recruit functional DCC in wild-type males (Demakova et al. 2003). Alternatively, the active genes located within the DCC gaps might serve as targets for the complex but cannot realize this ability probably due to the chromatin environment.
Whether the genes within puffs and DCC gaps undergo dosage compensation remains to be answered. It seems plausible to suggest that transcription upregulation could be not so essential for a subset of highly expressed genes. Nevertheless, whatever the reasons for highly expressed loci to escape association with DCC, these genes are probably dosage compensated, which was shown at least for Sgs4 and the Broad-Complex (Breen and Lucchesi 1986; Kaiser et al. 1986; Chiang and Kurnit 2003). If many active genes lack DCC-binding sites in the immediate vicinity, they might achieve dosage compensation by a yet unknown pathway. It is very possible that upregulation of active genes within DCC gaps and puffing regions might be achieved, at least to some extent, via DCC-mediated establishment of a more open chromatin structure of the whole male X, suggesting that DCC affects transcription indirectly. Site-specific localization of H4Ac16 probably initiates a cascade of molecular remodeling events resulting in diffuse appearance of the whole male X chromosome (Bone et al. 1994). Generally, such a chromatin state would facilitate the access of various transcription and replication factors. Accordingly, in females having ectopic dosage compensation induced, the DCC gap corresponding to the intercalary heterochromatin region on the polytene X demonstrated a greater extent of both polytenization and replication than in the wild type. This clearly correlated with the higher local concentrations of the DCC in neighboring areas (Alekseyenko et al. 2002). Thus, despite the fact that the DCC-mediated site-specific histone acetylation pattern correlates with an increase in transcription of the underlying sequences (Akhtar et al. 2000; Henry et al. 2001; Smith et al. 2001), we believe it would be more accurate to suggest that there is no common scenario of dosage compensation for all the X-linked genes. Also, the DCC pattern appears essentially permanent and displays only negligible variations, both in the course of larval development (our data) and in different tissues (Sass et al. 2003), which might point to the contribution of yet unidentified epigenetic factors in the establishment and maintenance of DCC binding. For example, the transcriptional activity of X-linked genes could govern DCC settling on the male X in early embryogenesis, and this pattern might be subsequently reproduced epigenetically. Hence, this scenario would imply high stability of the DCC pattern at least for the housekeeping genes, rather than dramatic changes in DCC distribution resulting from fine-tuned transcriptional programs further in development.
Future molecular studies of the dosage compensation status of active genes mapping to the DCC gaps could determine whether dosage compensation can also utilize some unknown mechanisms other than site-specific acetylation of H4 at lysine 16 leading to site-specific transcription enhancement. Alternatively, there may be many more X-linked genes whose expression does not require dosage compensation than was expected to date. Regardless, the important question remains how functional DCC recognizes its targets among the active genes on the male X chromosome.
Acknowledgments
We thank A. A. Gortchakov, Yu. B. Schwartz, and A. A. Alekseyenko for helpful discussions of our results. This work was supported by Fogarty International Research Collaboration Award (FIRCA) grant (International Cooperation Program) NI R03TW05669-01, Program for Scientific Schools RF 00-15-97984, and Program for Molecular and Cellular Biology NSH-918.2003.4.
References
- Akhtar, A., and P. B. Becker, 2000. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5: 367–375. [DOI] [PubMed] [Google Scholar]
- Akhtar, A., D. Zink and P. B. Becker, 2000. Chromodomains are protein-RNA interaction modules. Nature 407: 405–409. [DOI] [PubMed] [Google Scholar]
- Alekseyenko, A. A., O. V. Demakova, E. S. Belyaeva, G. F. Makarevich, I. V. Kotlikova et al., 2002. Dosage compensation and intercalary heterochromatin in X chromosomes of Drosophila melanogaster. Chromosoma 111: 106–113. [DOI] [PubMed] [Google Scholar]
- Andres, A. J., J. C. Fletcher, F. D. Karim and C. S. Thummel, 1993. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev. Biol. 160: 388–404. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., C. Chihara, P. Meltzer and G. Richards, 1974. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harbor Symp. Quant. Biol. 38: 655–662. [DOI] [PubMed] [Google Scholar]
- Bai, X., A. A. Alekseyenko and M. I. Kuroda, 2004. Sequence-specific targeting of MSL complex regulates transcription of the roX RNA genes. EMBO J. 23: 2853–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, B. S., M. Gorman and I. Marin, 1994. Dosage compensation in Drosophila. Annu. Rev. Genet. 28: 491–521. [DOI] [PubMed] [Google Scholar]
- Belyaeva, E. S., L. S. Korochkina, I. F. Zhimulev and N. K. Nazarova, 1974. Puff characteristics in the X-chromosome of D. melanogaster females. Cytology 16: 440–445. [PubMed] [Google Scholar]
- Bhadra, U., M. Pal-Bhadra and J. A. Birchler, 1999. Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics 152: 249–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., 1996. X chromosome dosage compensation in Drosophila. Science 272: 1190–1191. [PubMed] [Google Scholar]
- Bone, J. R., and M. I. Kuroda, 1996. Dosage compensation regulatory proteins and the evolution of sex chromosomes in Drosophila. Genetics 144: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone, J. R., J. Lavender, R. Richman, M. J. Palmer, B. M. Turner et al., 1994. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 8: 96–104. [DOI] [PubMed] [Google Scholar]
- Breen, T. R., and J. C. Lucchesi, 1986. Analysis of the dosage compensation of a specific transcript in Drosophila melanogaster. Genetics 112: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champlin, D. T., M. Frasch, H. Saumweber and J. T. Lis, 1991. Characterization of a Drosophila protein associated with boundaries of transcriptionally active chromatin. Genes Dev. 5: 1611–1621. [DOI] [PubMed] [Google Scholar]
- Chang, K. A., and M. I. Kuroda, 1998. Modulation of MSL1 abundance in female Drosophila contributes to the sex specificity of dosage compensation. Genetics 150: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C., and P. A. Sharp, 2003. RNA polymerase II accumulation in the promoter-proximal region of the dihydrofolate reductase and gamma-actin genes. Mol. Cell. Biol. 23: 1961–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, P. W., and D. M. Kurnit, 2003. Study of dosage compensation in Drosophila. Genetics 165: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakova, O. V., I. V. Kotlikova, P. R. Gordadze, A. A. Alekseyenko, M. I. Kuroda et al., 2003. The MSL complex levels are critical for its correct targeting to the chromosomes in Drosophila melanogaster. Chromosoma 112: 103–115. [DOI] [PubMed] [Google Scholar]
- Fagegaltier, D., and B. S. Baker, 2004. X chromosome sites autonomously recruit the dosage compensation complex in Drosophila males. PLoS Biol. 2: e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furia, M., P. P. D'Avino, S. Crispi, D. Artiaco and L. C. Polito, 1993. Dense cluster of genes is located at the ecdysone-regulated 3C puff of Drosophila melanogaster. J. Mol. Biol. 231: 531–538. [DOI] [PubMed] [Google Scholar]
- Gerber, M., J. Ma, K. Dean, J. C. Eissenberg and A. Shilatifard, 2001. Drosophila ELL is associated with actively elongating RNA polymerase II on transcriptionally active sites in vivo. EMBO J. 20: 6104–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S., J. C. Lucchesi and J. E. Manning, 1992. The non-dosage compensated LSP1-alpha gene of Drosophila melanogaster lies immediately downstream of the dosage compensated L12 gene. Mol. Gen. Genet. 233: 49–52. [DOI] [PubMed] [Google Scholar]
- Gilfillan, G. D., I. K. Dahlsveen and P. B. Becker, 2004. Lifting a chromosome: dosage compensation in Drosophila melanogaster. FEBS Lett. 567: 8–14. [DOI] [PubMed] [Google Scholar]
- Gonzy, G., G. V. Pokholkova, F. Peronnet, B. Mugat, O. V. Demakova et al., 2002. Isolation and characterization of novel mutations of the Broad-Complex, a key regulatory gene of ecdysone induction in Drosophila melanogaster. Insect Biochem. Mol. Biol. 32: 121–132. [DOI] [PubMed] [Google Scholar]
- Gorman, M., A. Franke and B. S. Baker, 1995. Molecular characterization of the male-specific lethal-3 gene and investigations of the regulation of dosage compensation in Drosophila. Development 121: 463–475. [DOI] [PubMed] [Google Scholar]
- Gu, W., P. Szauter and J. C. Lucchesi, 1998. Targeting of MOF, a putative histone acetyl transferase, to the X chromosome of Drosophila melanogaster. Dev. Genet. 22: 56–64. [DOI] [PubMed] [Google Scholar]
- Gu, W., X. Wei, A. Pannuti and J. C. Lucchesi, 2000. Targeting the chromatin-remodeling MSL complex of Drosophila to its sites of action on the X chromosome requires both acetyl transferase and ATPase activities. EMBO J. 19: 5202–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey, M., and D. Reinberg, 2003. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113: 429–432. [DOI] [PubMed] [Google Scholar]
- Henry, R. A., B. Tews, X. Li and M. J. Scott, 2001. Recruitment of the male-specific lethal (MSL) dosage compensation complex to an autosomally integrated roX chromatin entry site correlates with an increased expression of an adjacent reporter gene in male Drosophila. J. Biol. Chem. 276: 31953–31958. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz, D., S. M. Pinchin, J. Gausz, H. Gyurkovics, G. Bencze et al., 1979. Deletion mapping of two D. melanogaster loci that code for the 70,000 dalton heat-induced protein. Cell 17: 565–571. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Y. Wang, D. L. Walker, H. Dong, C. Conley et al., 1999. JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol. Cell 4: 129–135. [DOI] [PubMed] [Google Scholar]
- Kageyama, Y., G. Mengus, G. Gilfillan, H. G. Kennedy, C. Stuckenholz et al., 2001. Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J. 20: 2236–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, K., M. Furia and D. M. Glover, 1986. Dosage compensation at the sgs4 locus of Drosophila melanogaster. J. Mol. Biol. 187: 529–536. [DOI] [PubMed] [Google Scholar]
- Kaplan, C. D., J. R. Morris, C. Wu and F. Winston, 2000. Spt5 and spt6 are associated with active transcription and have characteristics of general elongation factors in D. melanogaster. Genes Dev. 14: 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, R. L., I. Solovyeva, L. M. Lyman, R. Richman, V. Solovyev et al., 1995. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81: 867–877. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., V. H. Meller, P. R. Gordadze, G. Roman, R. L. Davis et al., 1999. Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell 98: 513–522. [DOI] [PubMed] [Google Scholar]
- Kernan, M. J., M. I. Kuroda, R. Kreber, B. S. Baker and B. Ganetzky, 1991. napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell 66: 949–959. [DOI] [PubMed] [Google Scholar]
- Kireeva, M. L., W. Walter, V. Tchernajenko, V. Bondarenko, M. Kashlev et al., 2002. Nucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcription. Mol. Cell 9: 541–552. [DOI] [PubMed] [Google Scholar]
- Komarnitsky, P., E. J. Cho and S. Buratowski, 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14: 2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda, M. I., M. J. Kernan, R. Kreber, B. Ganetzky and B. S. Baker, 1991. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell 66: 935–947. [DOI] [PubMed] [Google Scholar]
- Lee, C. G., K. A. Chang, M. I. Kuroda and J. Hurwitz, 1997. The NTPase/helicase activities of Drosophila maleless, an essential factor in dosage compensation. EMBO J. 16: 2671–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Lis, J. T., W. Neckameyer, R. Dubensky and N. Costlow, 1981. Cloning and characterization of nine heat-shock-induced mRNAs of Drosophila melanogaster. Gene 15: 67–80. [DOI] [PubMed] [Google Scholar]
- Lyman, L. M., K. Copps, L. Rastelli, R. L. Kelley and M. I. Kuroda, 1997. Drosophila male-specific lethal-2 protein: structure/function analysis and dependence on MSL-1 for chromosome association. Genetics 147: 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, I., and B. S. Baker, 1998. The evolutionary dynamics of sex determination. Science 281: 1990–1994. [DOI] [PubMed] [Google Scholar]
- Meller, V. H., and M. I. Kuroda, 2002. Sex and the single chromosome. Adv. Genet. 46: 1–24. [DOI] [PubMed] [Google Scholar]
- Meller, V. H., P. R. Gordadze, Y. Park, X. Chu, C. Stuckenholz et al., 2000. Ordered assembly of roX RNAs into MSL complexes on the dosage-compensated X chromosome in Drosophila. Curr. Biol. 10: 136–143. [DOI] [PubMed] [Google Scholar]
- Oh, H., J. R. Bone and M. I. Kuroda, 2004. Multiple classes of MSL binding sites target dosage compensation to the X chromosome of Drosophila. Curr. Biol. 14: 481–487. [DOI] [PubMed] [Google Scholar]
- Orphanides, G., and D. Reinberg, 2000. RNA polymerase II elongation through chromatin. Nature 407: 471–475. [DOI] [PubMed] [Google Scholar]
- Palancade, B., and O. Bensaude, 2003. Investigating RNA polymerase II carboxyl-terminal domain (CTD) phosphorylation. Eur. J. Biochem. 270: 3859–3870. [DOI] [PubMed] [Google Scholar]
- Pal Bhadra, M., U. Bhadra, J. Kundu and J. A. Birchler, 2005. Gene expression analysis of the function of the male-specific lethal complex in Drosophila. Genetics 169: 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuti, A., and J. C. Lucchesi, 2000. Recycling to remodel: evolution of dosage-compensation complexes. Curr. Opin. Genet. Dev. 10: 644–650. [DOI] [PubMed] [Google Scholar]
- Park, Y., R. L. Kelley, H. Oh, M. I. Kuroda and V. H. Meller, 2002. Extent of chromatin spreading determined by roX RNA recruitment of MSL proteins. Science 298: 1620–1623. [DOI] [PubMed] [Google Scholar]
- Rastelli, L., and M. I. Kuroda, 1998. An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech. Dev. 71: 107–117. [DOI] [PubMed] [Google Scholar]
- Reenan, R. A., C. J. Hanrahan and G. Barry, 2000. The mle(napts) RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron 25: 139–149. [DOI] [PubMed] [Google Scholar]
- Richter, L., J. R. Bone and M. I. Kuroda, 1996. RNA-dependent association of the Drosophila maleless protein with the male X chromosome. Genes Cells 1: 325–336. [DOI] [PubMed] [Google Scholar]
- Ruiz, M. F., M. R. Esteban, C. Donoro, C. Goday and L. Sanchez, 2000. Evolution of dosage compensation in Diptera: the gene maleless implements dosage compensation in Drosophila (Brachycera suborder) but its homolog in Sciara (Nematocera suborder) appears to play no role in dosage compensation. Genetics 156: 1853–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass, G. L., A. Pannuti and J. C. Lucchesi, 2003. Male-specific lethal complex of Drosophila targets activated regions of the X chromosome for chromatin remodeling. Proc. Natl. Acad. Sci. USA 100: 8287–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, A., J. Werner, E. D. Andrulis, T. Nakayama, S. Hirose et al., 2003. Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science 301: 1094–1096. [DOI] [PubMed] [Google Scholar]
- Schwartz, B. E., and K. Ahmad, 2005. Transcriptional activation triggers deposition and removal of the histone variant H3.3. Genes Dev. 19: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, B. E., S. Larochelle, B. Suter and J. T. Lis, 2003. Cdk7 is required for full activation of Drosophila heat shock genes and RNA polymerase II phosphorylation in vivo. Mol. Cell. Biol. 23: 6876–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeshin, V. F., R. Artero, M. Perez-Alonso and V. V. Shloma, 1998. Electron microscopic in situ hybridization of digoxigenin-dUTP-labelled DNA probes with Drosophila melanogaster polytene chromosomes. Chromosome Res. 6: 405–410. [DOI] [PubMed] [Google Scholar]
- Semeshin, V. F., E. N. Andreyeva, V. V. Shloma, H. Saumweber and I. F. Zhimulev, 2002. Immunogold electron microscope localization of proteins in Drosophila polytene chromosomes: applications and limitations of the method. Chromosome Res. 10: 429–433. [DOI] [PubMed] [Google Scholar]
- Sharma, A., and S. C. Lakhotia, 1995. In situ quantification of hsp70 and alpha-beta transcripts at 87A and 87C loci in relation to hsr-omega gene activity in polytene cells of Drosophila melanogaster. Chromosome Res. 3: 386–393. [DOI] [PubMed] [Google Scholar]
- Smith, E. R., A. Pannuti, W. Gu, A. Steurnagel, R. G. Cook et al., 2000. The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol. Cell. Biol. 20: 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. R., C. D. Allis and J. C. Lucchesi, 2001. Linking global histone acetylation to the transcription enhancement of X-chromosomal genes in Drosophila males. J. Biol. Chem. 276: 31483–31486. [DOI] [PubMed] [Google Scholar]
- Stokes, D. G., K. D. Tartof and R. P. Perry, 1996. CHD1 is concentrated in interbands and puffed regions of Drosophila polytene chromosomes. Proc. Natl. Acad. Sci. USA 93: 7137–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckenholz, C., Y. Kageyama and M. I. Kuroda, 1999. Guilt by association: non-coding RNAs, chromosome-specific proteins and dosage compensation in Drosophila. Trends Genet. 15: 454–458. [DOI] [PubMed] [Google Scholar]
- Wang, Y., W. Zhang, Y. Jin, J. Johansen and K. M. Johansen, 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105: 433–443. [DOI] [PubMed] [Google Scholar]
- Weeks, J. R., S. E. Hardin, J. Shen, J. M. Lee and A. L. Greenleaf, 1993. Locus-specific variation in phosphorylation state of RNA polymerase II in vivo: correlations with gene activity and transcript processing. Genes Dev. 7: 2329–2344. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and F. Grosse, 2004. Multiple functions of nuclear DNA helicase II (RNA helicase A) in nucleic acid metabolism. Acta Biochim. Biophys. Sin. (Shanghai) 36: 177–183. [DOI] [PubMed] [Google Scholar]
- Zhimulev, I. F., 1999. Genetic organization of polytene chromosomes. Adv. Genet. 39: 1–589. [DOI] [PubMed] [Google Scholar]
- Zhimulev, I. F., E. S. Belyaeva, O. M. Mazina and M. L. Balasov, 1995. Structure and expression of the BR-C locus in Drosophila melanogaster, Diptera: Drosophilidae. Eur. J. Entomol. 92: 263–270. [Google Scholar]