Abstract
C. elegans mutants lacking the dense-core vesicle priming protein UNC-31 (CAPS) share highly similar phenotypes with mutants lacking a neuronal Gαs pathway, including strong paralysis despite exhibiting near normal levels of steady-state acetylcholine release as indicated by drug sensitivity assays. Our genetic analysis shows that UNC-31 and neuronal Gαs are different parts of the same pathway and that the UNC-31/Gαs pathway is functionally distinct from the presynaptic Gαq pathway with which it interacts. UNC-31 acts upstream of Gαs because mutations that activate the Gαs pathway confer similar levels of strongly hyperactive, coordinated locomotion in both unc-31 null and (+) backgrounds. Using cell-specific promoters, we show that both UNC-31 and the Gαs pathway function in cholinergic motor neurons to regulate locomotion rate. Using immunostaining we show that UNC-31 is often concentrated at or near active zones of cholinergic motor neuron synapses. Our data suggest that presynaptic UNC-31 activity, likely acting via dense-core vesicle exocytosis, is required to locally activate the neuronal Gαs pathway near synaptic active zones.
AT least two distinct vesicle systems, synaptic vesicles and dense core vesicles, mediate the presynaptic release of neurotransmitters. The synaptic vesicle system mediates the highly localized, rapid release of classical neurotransmitters from active zones. Presynaptic dense core vesicle release does not occur at morphologically defined active zones and is generally associated with neuropeptide release. Little is known about whether and how these two vesicle systems interact at the synapse during the execution of behaviors. Both vesicle systems release their contents in response to electrically induced calcium influx (Martin 2003), and each system has a mechanism for priming vesicles, which is the process by which vesicles become competent to fuse in response to the calcium signal. Synaptic vesicles use an UNC-13-based system (Aravamudan et al. 1999; Augustin et al. 1999; Richmond et al. 1999, 2001). Dense core vesicles use a CAPS-based system (Grishanin et al. 2004). UNC-13 and CAPS are distantly related as indicated by a shared UNC-13 homology domain (Grishanin et al. 2002).
In either system, if vesicles are not primed, they will not release neurotransmitter in response to stimuli, even if calcium enters the synapse or cell (Hay and Martin 1992; Aravamudan et al. 1999; Augustin et al. 1999; Richmond et al. 1999). Although the pathways regulating dense core vesicle priming are poorly understood, previous studies have shown that diacylglycerol (DAG) and cAMP can regulate the readily releasable (primed) pool of synaptic vesicles (Chen and Regehr 1997; Stevens and Sullivan 1998; Waters and Smith 2000; Kidokoro et al. 2004; Virmani et al. 2005). Genetic studies in the model organism Caenorhabditis elegans have identified a network of pathways that appears to regulate priming in the synaptic vesicle system. These studies have suggested a model, the synaptic signaling network, in which the integrated activities of at least three major Gα signaling pathways control synapse activation to regulate locomotion rate (Figure 1 and references in legend). The core pathway of this network is the Gαq pathway. It produces, among other possible signals, DAG. Animals lacking the Gαq pathway are almost completely paralyzed (Brundage et al. 1996), and yet briefly exposing such animals to an optimal concentration of phorbol esters quickly restores both movement and steady-state acetylcholine release to near wild-type levels (Reynolds et al. 2005). Phorbol esters are DAG analogs that are known to increase the pool of primed synaptic vesicles and the rate at which the pool refills (Stevens and Sullivan 1998; Waters and Smith 2000; Virmani et al. 2005). Genetic epistasis studies have shown that both the synaptic Gαq pathway and phorbol esters are strongly dependent on the UNC-13 priming protein to exert their effects (Lackner et al. 1999; Reynolds et al. 2005). The Gαq pathway is negatively regulated by the Gαo pathway by one or more unknown mechanisms, by a diacylglycerol kinase, and by the EAT-16 RGS protein (Hajdu-Cronin et al. 1999; Miller et al. 1999; Nurrish et al. 1999).
Figure 1.
Model of the synaptic signaling network. Solid lines indicate that direct interactions are known or likely, while dashed lines and/or large gaps between line endpoints and downstream effectors indicate predicted interactions or predicted missing components. Proteins that promote locomotion and/or neurotransmitter release are shown in green, while proteins that inhibit locomotion and/or neurotransmitter release are shown in red. This study addresses the relationship between the dense-core vesicle priming protein UNC-31 (CAPS) and the synaptic signaling network. See the text and the following references for details: Maruyama and Brenner (1991); Mendel et al. (1995); Segalat et al. (1995); Brundage et al. (1996); Koelle and Horvitz (1996); Hajdu-Cronin et al. (1999); Lackner et al. (1999); Miller et al. (1999, 2000); Nurrish et al. (1999); Richmond et al. (1999, 2001); Robatzek and Thomas (2000); Chase et al. (2001); van der Linden et al. (2001); Robatzek et al. (2001); Tall et al. (2003); Reynolds et al. (2005); and Schade et al. (2005).
The third major pathway in the synaptic signaling network is controlled by Gαs, which activates adenylyl cyclase to produce the cAMP second messenger. The first insights into the C. elegans neuronal Gαs pathway came from studies of transgenic strains overexpressing constitutively active Gαs and from observations of a gsa-1 (Gαs) null mutant (Korswagen et al. 1997; Berger et al. 1998). These studies found that too much Gαs pathway activity kills neurons via necrotic cell death, and that this killing can be blocked by mutations that reduce the activity of ACY-1 (adenylyl cyclase). This cell killing masks a role for the Gαs pathway in regulating locomotion rate and neurotransmitter release because strains in which the Gαs pathway is activated to a lesser degree (that does not kill neurons) have strongly hyperactive locomotion, and drug sensitivity assays indicate that they have increased acetylcholine release (Korswagen et al. 1997; Schade et al. 2005). Genetic studies suggest that the Gαs and Gαq pathways converge downstream of DAG production to regulate locomotion rate (Reynolds et al. 2005). Interestingly, although the neuronal Gαs pathway requires the Gαq pathway to exert its effects, it appears not to be required for synaptic vesicle priming, because animals lacking a neuronal Gαs pathway have normal levels of steady-state acetylcholine release, as indicated by aldicarb sensitivity assays (Reynolds et al. 2005). Similar results were reported for Drosophila Gαs null mutants, using electrophysiological methods to monitor glutamate release at neuromuscular junctions (Hou et al. 2003; Renden and Broadie 2003). However, despite their apparently normal steady-state acetylcholine release, C. elegans mutants lacking a neuronal GSA-1–ACY-1 pathway are nearly paralyzed (Korswagen et al. 1997; Schade et al. 2005). The near paralysis results from functional defects unrelated to nervous system development (Reynolds et al. 2005).
Identifying proteins that control activation of the neuronal Gαs pathway may provide insights into why animals lacking this pathway are paralyzed. In this study, we pursued this goal by searching for mutants with phenotypes similar to mutants lacking a neuronal Gαs pathway. After observing a neuronal-specific Gαs pathway null in a previous study (Reynolds et al. 2005), we noted its striking similarity to mutants lacking the presynaptic dense-core vesicle priming protein unc-31 (CAPS). In this study, we show that UNC-31 and neuronal Gαs are indeed different parts of the same pathway at synapses and that this pathway is functionally distinct from the Gαq pathway with which it interacts as part of the synaptic signaling network. UNC-31 acts upstream of Gαs because mutations that activate the Gαs pathway confer similar levels of strongly hyperactive, coordinated locomotion in both unc-31 null and (+) backgrounds. Using cell-specific promoters, we show that both UNC-31 and the Gαs pathway function in cholinergic motor neurons to regulate locomotion rate. Using immunostaining we show that UNC-31 is often concentrated at or near the active zones of cholinergic motor neuron synapses. Our data suggest that presynaptic UNC-31 activity, likely acting via dense-core vesicle exocytosis, is required to locally activate the neuronal Gαs pathway near synaptic active zones. The regulation of UNC-31 priming by as yet unknown signals may thus be a mechanism to control local activation of the neuronal Gαs pathway.
MATERIALS AND METHODS
Alleles:
unc-31(e928) represents a 4× outcrossed version of DA509 obtained from Leon Avery (Avery et al. 1993). egl-30(md186) is a reduction-of-function mutation (amino acid change D201E). Original references for other alleles used in this study are cited in the text and figure legends.
Analysis of the unc-31(e928) deletion:
We performed PCR of wild-type and e928 worm lysates as described (Miller et al. 2000). We tested different primer positions until we identified a primer pair flanking the deletion. We then sequenced the PCR product containing the deletion to determine the exact boundaries of the deletion.
Plasmids:
To make a full-length unc-31 cDNA, we reverse transcribed (StrataScript; Stratagene, La Jolla, CA) and amplified (Accuprime Pfx; Invitrogen, San Diego) an 1100-bp 5′ end fragment of the unc-31 coding region from wild-type C. elegans mRNA. We then fused the resulting cDNA fragment to the Kohara cDNA yk354a8, using a unique internal AatII site and an engineered NotI site in the 5′ primer to produce KG#104. To make KG#121 [rab-3∷acy-1(+) cDNA], we used Accuprime Pfx and primers engineered with restriction sites to amplify the 3780-bp unc-31 cDNA coding region from KG#104 and cloned the fragment into NheI/AgeI-cut KG#59 (a neuronal-specific C. elegans expression vector described in Schade et al. 2005). To make KG#65, an unc-17β∷ expression vector, we used Pfu Ultra Polymerase (Stratagene) and primers engineered with restriction sites to amplify the 0.5-kb unc-17β promoter from RM#605p (unc-17β∷GFP; gift of Jim Rand) and used this fragment to replace the myo-3 promoter in PstI/BamHI-cut pPD96.52 (muscle-specific C. elegans expression vector; gift of Andrew Fire). To make KG#126 [unc-17β∷unc-31(+) cDNA], we used NheI/AgeI to cut out the unc-31(+) cDNA from KG#121 and cloned it into the like-digested KG#65 unc-17β∷ expression vector. To make KG#84 [unc-17β∷acy-1 (P260S) gf cDNA], we used AgeI/XhoI to cut out the 3800-bp acy-1 (P260S) gf cDNA from KG#81 (Reynolds et al. 2005) and cloned it into the like-digested KG#65 unc-17β∷ expression vector. To make KG#122 (His6-UNC-31 N terminus for bacterial expression) we used Accuprime Pfx and primers engineered with restriction sites to clone the N-terminal 258 codons of unc-31 from KG#104 into NcoI/BamHI-cut pQE-60 (QIAGEN, Valencia, CA). In all constructs involving the cloning of PCR fragments, we used clones containing no mutations in the fragment of interest to establish the final plasmid stock.
Transgenes:
We produced transgenic strains bearing extrachromosomal arrays by the method of Mello et al. (1991). We used pBluescript carrier DNA to bring the final concentration of DNA in the injection mixture to 175 ng/μl. We made unc-31(e928); ceEx117 [rab-3∷unc-31(+) cDNA] by injecting unc-31(e928) mutants with KG#121 [rab-3∷unc-31(+) cDNA] at 35 ng/μl along with the cotransformation marker plasmids KG#68 [rab-3∷GFP] and KG#67 [ttx-3∷GFP] at 35 and 25 ng/μl, respectively. We made unc-31(e928); ceIs19 [unc-17β∷unc-31(+) cDNA] by injecting unc-31(e928) mutants with KG#126 [unc-17β∷unc-31(+) cDNA] at 75 ng/μl along with the cotransformation marker RM#605p [unc-17β∷GFP] at 25 ng/μl and integrating the resulting transgene. We made ceIs28 by injecting pha-1(e2123) animals with KG#84 [unc-17β∷acy-1 P260S gf cDNA] at 15 ng/μl along with the cotransformation marker plasmids RM#605p (50 ng/μl) and pBx [pha-1∷pha-1(+)] at 70 ng/μl and integrating the resulting transgene. We performed the integrations as described, except that cultures were screened for 100% transmittance of the GFP, not the pha-1 marker (Reynolds et al. 2005). The ceIs6 [myo-3∷acy-1 P260S gf cDNA], ceIs11 [rab-3∷acy-1 P260S gf cDNA], and pkIs296 [hsp-16-2∷gsa-1 Q208L gf] transgenes have been previously described (Korswagen et al. 1997; Reynolds et al. 2005).
Double mutants:
Unless otherwise specified, we constructed double mutants using standard genetic methods without additional marker mutations, and homozygosity of both mutations was confirmed by double-amplification PCR with nested primers (for deletion mutations) or by PCR and sequencing (for single-base change mutations). We verified the homozygous presence of the acy-1(pk1279) and unc-31(e928) deletions in all double mutants containing these mutations by double-amplification PCR, using nested primers as described (Reynolds et al. 2005). We constructed KG859 unc-31(e928) dpy-20(e1282); pkIs296 [HS∷ gsa-1 Q208L gf] using methods analogous to those used to construct the corresponding strain containing ric-8(md303) in place of unc-31(e928) (Reynolds et al. 2005). We collected larval-arrested acy-1(pk1279); unc-31(e928) double mutants for assays, documentation, and genotype verification as described for other pk1279-containing strains (Reynolds et al. 2005). We crossed the ceIs6, ceIs11, and ceIs28 transgenes into the unc-31(e928) background, using standard methods followed by nested PCR amplification to verify e928 homozygosity and confirmation of 100% transmittance of the GFP cotransformation marker. We produced unc-31(e928); ceIs19 by direct injection into unc-31(e928) mutants followed by integration of the resulting transgene. Reynolds et al. (2005) describes KG761 acy-1(pk1279); ceEx108 [myo-3∷acy-1(+) cDNA] and KG762 acy-1(pk1279); egl-30(tg26); ceEx108 [myo-3∷acy-1(+) cDNA].
Locomotion assays:
We performed standard locomotion assays as described, using standardized plates and assay procedures (Miller et al. 1999; Reynolds et al. 2005). We collected larval-arrested acy-1(pk1279); unc-31(e928) double mutants and egl-30(ad810) single mutants for assays, documentation, and genotype verification as described for other pk1279 and ad810-containing strains (Reynolds et al. 2005). We produced and assayed identically staged N2 (wild type) and single-mutant control animals in the same manner as the larval-arrested double mutants. We assayed egl-30(ad805); unc-31(e928) and egl-30(md186); unc-31(e928) double mutants and egl-30(ad810) single mutants by a track locomotion assay as described (Reynolds et al. 2005). We performed locomotion assays following heat-shock treatment as described (Schade et al. 2005), with the heat-shock and recovery times specified in Figure 7. We performed locomotion assays of phorbol ester-treated animals as described, using the 5-μm phorbol myristate acetate (PMA) concentration and the 2.5-hr preexposure time described as optimal for adult animals (Reynolds et al. 2005).
Figure 7.
The activated Gαs pathway suppresses the paralysis of unc-31 null mutants via a highly specific, short timescale mechanism. (A) Activating the Gαs pathway does not strongly rescue the paralysis of Gαq reduction-of-function mutants that are paralyzed to a similar degree as unc-31 nulls. Shown are the mean locomotion rates, expressed as body bends per minute. The first group of bars on the left represents wild-type and single-mutant control strains, which are color coded in the inset. Subsequent groups of bars show strains homozygous for unc-31(e928) or two different egl-30 (Gαq) reduction-of-function mutants as indicated on the x-axis. The first bar in each of these latter groups represents the single mutant that is indicated on the x-axis, while subsequent bars in each group represent double mutants in which the second mutation activates a component of the Gαs pathway as ordered in the inset. The mutations in the inset activate ACY-1 (adenylyl cyclase) or protein kinase A [the latter is an indirect activation via strongly reducing the function of the KIN-2 regulatory subunit, as described (Reynolds et al. 2005)]. Error bars represent the standard error of the mean for 8–10 animals. Gaps between bars in the egl-30 groups represent double mutants that have not been constructed. The values for the single mutants acy-1(md1756), acy-1(ce2), and kin-2(ce179) are reprinted with permission from Schade et al. (2005) for comparison. (B) Activating the Gαs pathway suppresses the paralysis of unc-31 null mutants via a short timescale that is not dependent on development. Shown are the mean locomotion rates, expressed as body bends per minute. Dark and light blue bars indicate locomotion rates without or with heat-shock treatment, respectively. The pkIs296 transgene used in this experiment was produced in a previous study (Korswagen et al. 1997). Error bars represent the standard error of the mean for populations of 8 animals. The values for the pkIs296 single mutant (plus or minus heat shock) are reprinted with permission from Schade et al. (2005) for comparison.
Video production and imaging:
We captured and converted videos of worms on agar plates containing OP-50 bacterial lawns as described (Schade et al. 2005). We collected still images of mutants, using an Olympus C3040 digital camera mounted on an Olympus SZX-12 stereomicroscope. We obtained high-magnification larval images, using a high-resolution 2×, 0.275 NA Plan apochromatic objective.
Drug sensitivity assays:
The dose-response curves in Figures 3D, 5, C and D, and 6C all represent aldicarb assays performed by the population growth rate method (Miller et al. 1999). We typically choose the population growth rate method when one or more of the strains in the group are too paralyzed in the absence of the drug to use the paralysis aldicarb assay described below, although for most strains in this article we have done both kinds of aldicarb assays. Each data point in the population growth rate aldicarb assays typically represents 300–900 progeny and is representative of duplicate experiments done in parallel. Because of the time-consuming nature of this assay, and because we show the full dose response over a wide concentration series, we do not do these experiments in triplicate and thus cannot show error bars. We chose only larvae that were uniformly GFP positive in body wall muscle cells for the acy-1(pk1279); ceEx108 transgenic animals in Figure 3. We performed aldicarb paralysis assays on solid media as described (Lackner et al. 1999; Nurrish et al. 1999), with the modifications listed below. We do these paralysis assays in triplicate and thus show error bars (Figures 5E and 6D). We added aldicarb to a final concentration of 2 mm from a 10-mm stock solution in ddH2O (allowing ∼2–3 hr for dissolving the aldicarb before adding to the 55° cooled molten media), and we made the media with 20% less water than normal to compensate for the large drug volume. We seeded aldicarb-containing plates with OP-50 on the day they were poured and stored them at room temperature for 2 days, lid side up, before using. We used 1 mm aldicarb in the Figure 5E assays because the phorbol ester causes strong aldicarb hypersensitivity. We preincubated animals designated for plates containing PMA + aldicarb with 5.0 μm PMA to allow the PMA time course to maximize before beginning the aldicarb + PMA time course. We produced these phorbol ester plates as described (Schade et al. 2005). The PMA preexposure time was 2.5 hr (based on pretesting to determine the maximal response of each strain). We produced three such preincubation plates at staggered times, so they would be available for each of the three independent trials of the aldicarb paralysis assay. We used 20 animals for each strain/condition per trial. We performed body wall muscle contraction assays as described (Reynolds et al. 2005).
Figure 3.
UNC-31 (CAPS) null mutants and mutants lacking a neuronal Gαs pathway share similar phenotypes. Note that for purposes of clarity, Gαq, Gαs, and Gαo are at times referred to as Gq, Gs, and Go in this and subsequent figures. (A) unc-31 null mutants look similar to mutants that lack a neuronal Gαs pathway when viewed on culture plates through a stereomicroscope. Images compare a typical N2 (wild-type) animal with a typical unc-31(e928) null mutant and a mutant that lacks a neuronal Gαs pathway. The latter's genotype is acy-1(pk1279); ceEx108 [myo-3∷acy-1(+) cDNA], which expresses acy-1 (adenylyl cyclase) only in muscle cells and thus is null for acy-1 in the nervous system (Reynolds et al. 2005). All three animals are feeding in a lawn of bacteria. Note the similar straight posture associated with the paralysis of the unc-31 and neuronal Gαs pathway null mutants. The image “Mutant lacking a neuronal Gαs pathway” is reprinted with permission from Reynolds et al. (2005) for comparison. (B) unc-31 and neuronal Gαs pathway null mutants exhibit a similar degree of paralysis. Shown are the mean locomotion rates, expressed as body bends per minute, of the indicated strains. An egl-30 (Gαq) reduction-of-function mutant (ad805) (Brundage et al. 1996) that is paralyzed to a similar extent as mutants lacking a neuronal Gαs pathway or UNC-31 is shown for comparison. unc-31 null mutants are only slightly less paralyzed than animals that lack a neuronal Gαs pathway. The bar on the right demonstrates that a transgenic array containing the unc-31 (+) cDNA expressed in the nervous system fully rescues the locomotion defect of the unc-31(e928) null. Error bars represent the standard error of the mean for populations of 10 animals. Transgenic arrays are ceEx108 [myo-3∷acy-1(+) cDNA (expressed only in muscle)] and ceEx117 [rab-3∷unc-31(+) cDNA (expressed only in the nervous system)]. The value for acy-1(pk1279); ceEx108 is reprinted with permission from Reynolds et al. (2005) for comparison. (C) unc-31 and neuronal Gαs pathway null mutants respond normally to the cholinergic agonist levamisole. The graph depicts the contraction of individual animals after a 20-min exposure to various concentrations of levamisole. Error bars represent the standard errors for six individual animals at each concentration. acy-1(pk1279); ceEx108 (a transgenic muscle-rescued acy-1 null mutant) was used as the neuronal Gαs pathway null. Note that the levamisole sensitivity of unc-31(e928) is indistinguishable from wild type, while the neuronal Gαs pathway null appears only slightly hypersensitive to levamisole (although this is not statistically significant at any concentration). We did not investigate the extent to which overexpression of the acy-1(+) transgene cDNA in muscle may have contributed to the slight levamisole hypersensitivity, as has been previously shown to occur with muscle-specific expression of acy-1(gf) (Schade et al. 2005). Statistical significance tests used the unpaired t-test with Welch correction, with the level of statistical significance set at P = 0.05. The data for N2 and the neuronal Gs pathway null are reprinted with permission from Reynolds et al. (2005) for comparison and were generated as part of the same experiment with the unc-31 null. (D) unc-31 and neuronal Gαs pathway null mutants exhibit near-normal aldicarb sensitivity. Shown are the population growth rates of strains on various concentrations of the acetylcholinesterase inhibitor aldicarb. One hundred percent represents the number of progeny produced from a starting population of L1 larvae over a 96-hr period in the absence of aldicarb. Curves are representative of duplicate experiments. Complete genotypes are as follows: unc-31 null, unc-31(e928); Gαq reduction-of-function mutant, egl-30(ad805) (Brundage et al. 1996); and neuronal Gs pathway null, acy-1(pk1279); ceEx108 (a transgenic muscle-rescued acy-1 null mutant) (Reynolds et al. 2005). The data for N2, egl-30(ad805), and the neuronal Gs pathway null are reprinted with permission from Reynolds et al. (2005) for comparison and were generated as part of the same experiment with the unc-31 null.
Figure 5.
Epistasis analysis suggests that the neuronal Gαs pathway is downstream of UNC-31 and functionally inactive in unc-31 nulls. (A) Circuit model of the Gαq and Gαs branches of the synaptic signaling network depicting the components and mutations used in this experiment. “Blinker marks” next to Gαq and Gαs signify the strong gain-of-function mutations used in this experiment, while the “X” through UNC-31 indicates the unc-31 null background into which we transferred each gain-of-function mutation. (B) The paralysis of unc-31 nulls is converted to hyperactive locomotion by a mutation that activates the Gαs pathway, and activating the Gαq pathway in the unc-31 null confers a phenotype similar to activating the Gαq pathway in a neuronal Gαs pathway null. Shown are the mean locomotion rates, expressed as body bends per minute, of various strains. The first group of three bars on the left represents wild-type and single-mutant control strains, which are color coded in the inset. In the right two sets of bars, double- and single-mutant strains homozygous for unc-31(e928) or acy-1(pk1279); ceEx108 (a strain lacking a neuronal Gαs pathway) are grouped together as indicated. Note that activating the Gαs pathway in the unc-31 null results in a locomotion rate nearly as hyperactive as the gsa-1 gain-of-function single mutant, whereas activating the Gαq pathway in either an unc-31 null background or a background lacking a neuronal Gαs pathway results in an approximately wild-type locomotion rate along with a tendency to knot or coil (photos). Error bars represent the standard error of the mean for 8–10 animals. A portion of the data in this graph is reprinted with permission from Reynolds et al. (2005) for comparison. See also supplemental QuickTime movies (http://www.genetics.org/supplemental/). (C and D) Activating either the Gαs pathway or the Gαq pathway in the unc-31 null mutant causes hypersensitivity to aldicarb. Shown are the population growth rates of strains on various concentrations of the acetylcholinesterase inhibitor aldicarb. One hundred percent represents the number of progeny produced from a starting population of L1 larvae over a 96-hr period in the absence of aldicarb. Each strain's response, including the wild-type control, is indicated. gsa-1(gf), gsa-1(ce81); egl-30(gf), egl-30(tg26); and unc-31(0), unc-31(e928). The data for gsa-1(ce81) are reprinted with permission from Schade et al. (2005) for comparison. (E) Phorbol esters induce identical aldicarb hypersensitivity in wild-type and unc-31 null mutants. Shown is a graph depicting the percentage of animals that are paralyzed, over a time course, on plates containing 1 mm aldicarb and/or 5 μm phorbol myristate acetate. Strains and conditions are indicated with arrows. Note that both wild-type and unc-31 null mutants that have been treated with phorbol ester are equally hypersensitive to the paralytic effects of aldicarb (their lines overlap over most of the time course), which suggests that they are both releasing similar amounts of acetylcholine. Error bars represent the standard error of the mean for three independent populations of 20 animals each.
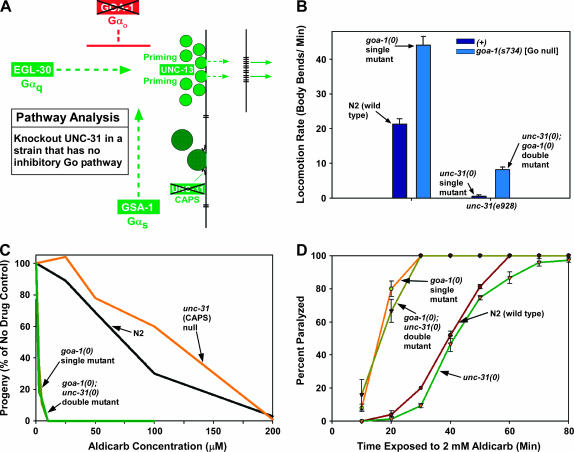
Figure 6.
Knocking out the inhibitory Gαo pathway affects the unc-31 null in a manner similar to activating the Gαq pathway or to phorbol ester treatment. (A) Circuit model of the synaptic signaling network depicting the components and mutations used in this experiment. The X's through GOA-1 and UNC-31 indicate the null goa-1 and unc-31 mutations used in this experiment, which were goa-1(sa734) and unc-31(e928). (B) Knocking out the inhibitory Gαo pathway only partially suppresses the paralysis of the unc-31 null. Shown are the mean locomotion rates, expressed as body bends per minute. The bars on the left represent wild-type and the goa-1 null single-mutant control strains, while the bars on the right represent strains containing the unc-31 null mutation. Note that knocking out the Gαo pathway improves the locomotion rate of the unc-31 null only to a level that is ∼18% of the goa-1 null single mutant and ∼40% of wild type. Error bars represent the standard error of the mean for 8–10 animals. The value for goa-1(sa734) is reprinted with permission from Reynolds et al. (2005) for comparison. (C and D) Knocking out unc-31 does not affect the strong aldicarb hypersensitivity caused by knocking out the Gαo pathway. (C) The population growth rates of strains on various concentrations of aldicarb. One hundred percent represents the number of progeny produced from a starting population of L1 larvae over a 96-hr period in the absence of aldicarb. Note that the strong aldicarb hypersensitivity of the goa-1(0) single mutant is closely matches that of a double mutant that is null for both unc-31 and goa-1, despite the rather sluggish locomotion rate of this double mutant (B). (D) The acute response of these strains to a fixed concentration of 2 mm aldicarb over an 80-min time course (using paralysis as the endpoint). Error bars represent the standard errors of populations of 25 animals at each time point.
Antibodies:
To produce the UNC-31-specific antibody KM16C-3.1, we prepared a recombinant UNC-31 fragment by first transforming KG#122 (His6-UNC-31[1-258]; see Plasmids) into the bacterial expression host BL21-DE3. We grew a 5-liter culture to an A600 of 0.4, lowering the incubation temperature gradually during growth such that the 0.4 OD culture was at 19°. We then added IPTG to 30 μm and continued growth for ∼17 hr before harvesting the cells. Cell pellets were frozen in liquid nitrogen and rapidly thawed before resuspending in 67 ml ice-cold lysis buffer [50 mm Tris–HCl, pH 8.0/10% glycerol/2 mm β-mercaptoethanol/0.1 mm PMSF/1 μg/ml pepstatin/1 μg/ml leupeptin/0.255 mg/ml lysozyme (Sigma, St. Louis)] per liter of culture. All steps hereafter were at 4°. To lyse, we stirred the suspension for 30 min, then added 5 mg of DNAse (Sigma D-4527) and 400 μl of 1 m MgSO4, and incubated 30 min more with stirring. After clearing the lysate with a 30-min 33,000 × g spin, we added NaCl to 300 mm and loaded the lysate at 0.5 ml/min onto a 5-ml Ni-NTA agarose (QIAGEN) column hooked to a Biologic (Bio-Rad, Hercules, CA) low pressure chromatography system. We washed the column with 100 ml 47 mm Tris–HCl, pH 8.0/50 mm NaCl/10% glycerol/5 mm imidazole and then eluted with a 50-ml gradient of 5–500 mm imidazole in the same buffer. We analyzed peak fractions by SDS–PAGE and determined that a band of the correct size was ∼90% pure. We further purified the protein on an SDS–PAGE gel and homogenized the Coomassie-stained gel band in a 10-ml Kontes Potter-Elvehjem tissue grinder such that the final concentration was 330 μg/ml of gel suspension. We sent the suspension to Cocalico (Cocalico, PA) for injection into a goat (660 μg + 2 × 200-μg boosts at 4-week intervals). We affinity purified UNC-31-specific antibodies by incubating 4 ml of serum with nitrocellulose strips containing 1500 μg of SDS–PAGE-purified UNC-31 fragment, using a previously described method (Miller et al. 2000).
Janet Duerr provided the UNC-17-specific antibody mAb1403 (as mouse monoclonal ascites) (Duerr et al. 2001), and Mike Nonet provided the UNC-10-specific antibody RIM5431 (as rabbit serum) (Koushika et al. 2001).
Immunostaining:
We developed a new immunostaining procedure for this study. We rinsed larvae from freshly starved plate cultures, plated them at a density of 4500 animals per 60-mm spread plate, and grew them for 2 days at room temperature to produce young adults. We harvested animals with ice-cold M9 buffer and used 3′ × 1500-rpm clinical centrifuge spins to concentrate the animals and washed them three times with 10 ml of M9 buffer, followed by resuspension in 300 μl/9000 animals. We applied 50 μl (∼1500 animals) of the worm suspension onto a clean glass slide and placed another slide on top of the first one (offset) such that the worms spread out between the slides in a thin layer. We placed the slide sandwich on a block of dry ice to freeze for ≥20 min and then repeated this for the remaining animals in the suspension. We prepared 40 ml of 4% formaldehyde (made from Ted Pella EM grade 16% formaldehyde) in 1× PBS in a 50-ml conical tube and cooled it on ice for ≥1 hr. We made four pairs of dummy slide sandwiches containing only ddH2O and froze these on dry ice for ≥20 min. We transferred the fixative from ice to room temperature and then cracked the four pairs of frozen dummy sandwiches into the fixative to precool it and make it slushy. To do this, we cracked the slides apart one pair at a time, placed them back to back, dipped them into the fixative, and rinsed/scraped off the resulting sheets of ice with a Pasteur pipette, before discarding the rinsed slide pair. We then vigorously cracked the experimental slide sandwiches one pair at a time in a similar manner, maintaining the fixative in a semifrozen, slushy state. We rocked the slushy fixative worm suspension until the slush just melted and then collected the worms by pouring them onto the glass frit of a 4-ml Econo-Column (Bio-Rad) with a funnel parafilmed to the top. We drained the fixative down to ∼1 ml, stopped the flow, and used a blunted 1-ml pipette tip to transfer the suspension to a microfuge tube. We quickspun the suspension and replaced the cold fixative with 1 ml of room temperature fixative. After a 30-min incubation at room temperature, we spun the suspension for 2 min at 12,000 rpm in a JA18.1 rotor at 2° and aspirated the fixative. We performed all subsequent steps at room temperature. We then added 1 ml of 0.1 m glycine, pH 7.4, and incubated 5 min to quench the reaction. After a quickspin in the microfuge, we washed the worms briefly with the glycine solution, followed by a quickspin and aspiration of supernatant. We then washed the worms with 3 × 400 μl of antibody buffer B (ABB: 1× PBS/0.5% Triton X-100/1 mm EDTA/0.1% BSA/0.05% sodium azide), with the wash times determined by the time it takes the animals to settle at 1 × g (a few minutes). We blocked animals 1 hr with 75 μl of ABB + 3% BSA per 4500 animals. We mixed primary antibodies in ABB + 3% BSA at two times the final concentration needed and mixed them 1:1 with the worm suspension in the blocking tubes. After an overnight incubation, we pelleted worms with a quickspin, vacuumed off the primary solution, and washed them four times with ABB, with the wash volume being two times the volume of the primary one. To the worm suspensions, we then added 200 μl/3000 animals of the appropriate secondary antibodies diluted to 1.8 μg/ml and incubated them 4 hr protected from light. We then washed the worms as above, followed by two washes with PBS, and then reduced the volume of the suspension to 15 μl/1500 animals, added an equal volume of mounting medium (20 mg/ml n-propyl gallate/30 mm Tris, pH 9.0/70% glycerol), and mounted 30 μl of the suspension per slide using a 24 × 30-mm coverslip sealed with nail polish.
We used the primary antibodies at the following dilutions: KM16C-3.1 (α-UNC-31) 1/200; mAb1403 (α-UNC-17) 1/5000 from ascites; and RIM5431 (α-UNC-10) 1/12,000. We produced secondary antibodies by coupling whole IgG [Jackson ImmunoResearch (West Grove, PA) “ML” quality for multilabeling] to Alexa 555 or Alexa 647 (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. We purchased Cy2-coupled secondaries directly from Jackson ImmunoResearch.
We collected fluorescent images using a Nikon Eclipse TE2000-E inverted microscope equipped with a 60× 1.4 NA oil planapochromat objective (CF160 type), a 1.5× tube lens, a motorized linear-encoded z-drive, and a motorized filter turret containing GFP, Cy3, and Cy5 image-registered filter cubes (Semrock). Our illumination source was an X-Cite 120 illuminator (EXFO), and we captured images with an ORCA-AG camera (Hamamatsu, Bridgewater, NJ) controlled by Metamorph Premier software. To preserve quantitative information, we chose exposure times in which the maximum pixel intensity did not exceed 90% of the dynamic range of the camera. We collected all images from animals in which the nerve cord of interest was oriented up and close to the surface of the coverslip, and all images are maximum projections of a z-series taken through the entire cord, including most of the out-of-focus light, using a step size of 140 nm.
We used AutoDeblur Gold CWF to deconvolve image stacks, using the Adaptive PSF Blind Method and 20 iterations. After deconvolving, we used AutoDeblur Gold CWF to double the xy size of the images to reduce pixel size, using the suggested ideal linear interpolation algorithm to maintain quantitative accuracy. We applied pixel shifts of ∼54 nm in the x and y directions to the final overlaid images to reflect a small amount of chromatic aberration inherent to the system (in the center of the field of view), which we determined experimentally by triple staining UNC-10 active zones with three different color secondary antibodies.
Image analysis and quantification:
We performed all quantitative analysis on 16-bit files after deconvolution and resizing. We quantified synaptic and subsynaptic regions over a 30-μm length of ventral nerve cord, using the Metamorph Premier software package. We traced regions freehand, using the trace region tool followed by automatic logging of measurements to an excel spreadsheet and further calculations to subtract background and calculate UNC-31/UNC-10 ratios as indicated in the supplemental Tables 1 and 2 legends (http://www.genetics.org/supplemental/).
RESULTS
UNC-31 (CAPS) null mutants and mutants lacking a neuronal Gαs pathway share similar phenotypes:
A previous study determined that the unc-31(e928) mutant has a large deletion in the unc-31 gene (Livingstone 1991). When compared to other unc-31 alleles, e928 acts like a strong reduction-of-function or null mutation (Avery et al. 1993). Our analysis of the e928 deletion shows it is a null, because it removes over half of the protein, including half of the PH domain, all of the highly conserved UNC-13 homology domain, and the C-terminal dense-core vesicle binding domain defined by Grishanin et al. (2002) (Figure 2). In addition, the deletion extends 290 bp past the 3′ end of the untranslated region, thus, in all likelihood, eliminating the polyadenylation signal and any other 3′ control sequences that are likely necessary for producing a stable mRNA.
Figure 2.
Conserved UNC-31 (CAPS) domains and a null mutation. Shown is a scale drawing of C. elegans UNC-31 depicting the known domains of CAPS (its vertebrate ortholog), as defined by Grishanin et al. (2002). The pleckstrin homology (PH) domain (489–596 of UNC-31) mediates specific interactions with the plasma membrane, while a C-terminal domain (1078–1260) mediates association with dense-core vesicles (Grishanin et al. 2002). A C2 domain (367–461) identified by Grishanin et al. (2002) is of unknown function. Percentages indicate the percent identity to rat CAPS (GenBank no. U16802). Note that the region of highest homology is the UNC-13 homology domain (domain DUF1041, using the NCBI conserved domain search tool; 696–892). A core subregion (734–815) of the UNC-13 homology domain is 84% identical to rat CAPS-1. The overall identity between UNC-31 and rat CAPS-1 is 49%. The region deleted by the unc-31(e928) mutation, as indicated, includes the entire C-terminal half of UNC-31 (553–1260 of 1260 aa total). This analysis is based on UNC-31 protein sequence in Wormbase, freeze version 130. All amino acid positions refer to C. elegans UNC-31.
Moorman and Plasterk (2002) produced the acy-1 (adenylyl cyclase) null mutation acy-1(pk1279) and showed that it causes larval growth arrest and paralysis. We recently showed that the larval growth arrest, but not the paralysis, of the acy-1 null mutant could be rescued by expressing the acy-1 cDNA just in body wall muscle cells (Reynolds et al. 2005). Such “muscle-rescued” acy-1 nulls (null for acy-1 in the nervous system) become healthy but paralyzed adults. We also showed that a constitutively active GSA-1 (Gαs) mutation exerts all of its effects on regulating locomotion rate through ACY-1 (Schade et al. 2005). Therefore, muscle-rescued acy-1 nulls effectively lack the neuronal Gαs pathway that regulates locomotion rate.
We observed that null unc-31 mutants, like mutants lacking a neuronal Gαs pathway, exhibit relatively straight, paralyzed postures on culture plates and locomotion rates that are ∼2–3% of the wild-type rate (Figure 3, A and B). These defects are caused solely by lack of functional UNC-31 (CAPS) in the nervous system, because a transgene carrying the unc-31(+) cDNA under control of the pan-neural rab-3 promoter restores a wild-type locomotion rate and confers posture, coordination, and egg-laying characteristics that are indistinguishable from wild type (Figure 3B; data not shown). The paralysis of unc-31 and neuronal Gαs pathway null mutants is not caused by decreased ability of their body wall muscles to respond to acetylcholine, because both mutants showed normal contraction of their body wall muscle cells in response to the cholinergic agonist levamisole (Figure 3C). We next asked if decreased acetycholine release contributes significantly to their paralysis by quantifying their sensitivity to the acetylcholinesterase inhibitor aldicarb. Since the secreted acetylcholine that accumulates in the presence of aldicarb is toxic, mutations that decrease or increase the steady-state rate of acetylcholine release confer resistance or hypersensitivity to aldicarb, respectively (Rand and Nonet 1997). In multiple independent assays, unc-31 null mutants showed only moderate resistance to low and medium concentrations of aldicarb, but, like wild-type animals and mutants lacking a neuronal Gαs pathway, growth halted on aldicarb concentrations of ≥200 μm (Figure 3D). This indicates that unc-31 null mutants have near-normal levels of steady-state acetylcholine release. In contrast, a similarly paralyzed egl-30 (Gαq) reduction-of-function mutant appears to have strongly decreased steady-state acetylcholine release, because it exhibits robust growth on aldicarb concentrations up to 400 μm (Figure 3D) and continues to grow and reproduce on concentrations up to 1600 μm (data not shown). These data suggest that the paralysis of unc-31 null mutants, as well as of mutants lacking a neuronal Gαs pathway, is caused by disruption of a process or pathway that is functionally distinct from the presynaptic Gαq pathway.
UNC-31 functions in the same pathway as Gαs to regulate locomotion rate:
If, as their similar phenotypes suggest, UNC-31 and Gαs are just two parts of the same pathway at synapses, then knocking out UNC-31 in a strain that has no Gαs pathway should have no further effect on locomotion rate. Figure 4B confirms this prediction. Knocking out either UNC-31 or the Gαs pathway results in similar levels of locomotion (∼2–3% of wild type), but knocking out both has no further effect on locomotion rate. From this result, we conclude that UNC-31 functions in the same pathway as Gαs to regulate locomotion rate. We also conclude that, in the absence of UNC-31, the neuronal Gαs pathway is essentially nonfunctional, because knocking out the neuronal Gαs pathway in an unc-31 null background had no further effect.
Figure 4.
In the synaptic signaling network, UNC-31 (CAPS) and Gαs function together in a pathway that is functionally distinct from the Gαq pathway. (A) Circuit model of the synaptic signaling network, depicting the components and mutations used in this experiment. The X's through the Gαs pathway and UNC-31 indicate null acy-1 (adenylyl cyclase) and unc-31 (CAPS) mutations, while the dashed X through EGL-30 represents the reduction-of-function egl-30 (Gαq) mutations that were combined with the unc-31 null in this experiment. (B) UNC-31 (CAPS) and neuronal Gαs are part of the same pathway. Shown are the mean locomotion rates, expressed as body bends per minute, of various strains labeled with arrows. The first group of bars on the left represents wild type and the unc-31 null single mutant. Subsequent groups of bars show strains homozygous for acy-1(pk1279) or two different egl-30 (Gαq) reduction-of-function mutants as indicated. The first bar in each of these latter groups represents the single mutant that is indicated on the x-axis, while subsequent bars in each group represent double mutants in which the second mutation is the unc-31 null mutation. The final bar on the right shows an egl-30 (Gαq) null single mutant. Error bars represent the standard error of the mean for 10 animals. Note that most of the wild-type bar is out of range of the graph. The values for acy-1(pk1279) and egl-30(ad810) are reprinted with permission from Reynolds et al. (2005) for comparison.
Could UNC-31 also function as part of the EGL-30 (Gαq) pathway for regulating locomotion rate? As noted above, our phenotypic comparison of unc-31 and egl-30 loss-of-function mutants suggests that they function in distinct pathways, because similarly paralyzed unc-31 and egl-30 mutants have widely differing aldicarb sensitivities. To further investigate the relationship of UNC-31 to the Gαq pathway, we examined the effect of reducing egl-30 (Gαq) pathway activity in an unc-31 null background. When we reduced the activity of the Gαq pathway by either one of two mutations and then made doubles with the unc-31 null, the locomotion rates of the doubles were much worse than those of the single mutants (Figure 4B). For example, the egl-30(md186) mutant is approximately six times more active than the unc-31 null, and yet its locomotion rate is reduced >200-fold when moved into the unc-31 null background. This synthetic effect is so severe that the locomotion rates of both of the egl-30; unc-31 double mutants we made are like that of the egl-30(ad810) mutant, which is null for the EGL-30 (Gαq) pathway (Brundage et al. 1996) (∼1 body bend/45 min) (Figure 4B). The simplest explanation for this strong synthetic interaction is that it is caused by simultaneous disruption of the Gαq pathway (through the egl-30 reduction-of-function mutations) and functional elimination of the Gαs pathway (through the unc-31 null mutation), since both pathways function nonredundantly to drive the locomotion behavior. An alternative explanation is that the unc-31 null mutation also reduces the function of the egl-30 (Gαq) pathway (in addition to functional elimination of the Gαs pathway) and that further reduction of egl-30 function by egl-30 reduction-of-function mutations causes the strong synthetic effect that we observed. However, in addition to the aldicarb comparisons presented earlier, forthcoming data in this article provide further evidence suggesting that the function of presynaptic UNC-31 is specific for the Gαs pathway.
The neuronal Gαs pathway is downstream of UNC-31:
To investigate the upstream/downstream relationship of UNC-31 (CAPS) to the Gαs pathway, we first analyzed double mutants containing the unc-31 null in combination with a mutation that strongly activates the Gαs pathway. The native gain-of-function mutation gsa-1(ce81) mutates the catalytic arginine that is required for GTP hydrolysis, thereby causing constitutive Gαs activation (Schade et al. 2005). We found that this mutation strongly rescues the paralysis of unc-31 nulls. Indeed turning on the Gαs pathway via this mutation confers similar levels of strongly hyperactive locomotion in both unc-31 null and (+) backgrounds (Figure 5B and supplemental movies at http://www.genetics.org/supplemental/). These double mutants had locomotion rates that were about double that of wild type and 82% of that of the gsa-1(ce81) gain-of-function single mutant. In addition, the hyperactive locomotion of these double mutants is highly coordinated, as is the case for gsa-1(ce81) single mutants (Figure 5 and supplemental movies at http://www.genetics.org/supplemental/). Combined with our earlier phenotypic comparisons and our results showing that UNC-31 and Gαs are two parts of the same pathway, we conclude from these epistasis results that UNC-31 directly or indirectly functions as a required upstream activator of the Gαs pathway.
Both UNC-31 and the neuronal Gαs pathway have the same relationship to the presynaptic Gαq pathway:
We next further investigated the relationship of UNC-31 (CAPS) to the Gαq pathway. To do this, we analyzed double mutants containing the unc-31 null in combination with the egl-30(tg26) mutation, which strongly activates signaling in the Gαq pathway (Doi and Iwasaki 2002; Natochin and Artemyev 2003; Reynolds et al. 2005) and confers a locomotion rate not significantly different from that of the gsa-1(ce81) single mutants used in the above epistasis experiment. We found that activating the Gαq pathway in this way greatly improved the locomotion rate of the unc-31 null, to approximately wild-type levels (Figure 5B and supplemental movies at http://www.genetics.org/supplemental/); however, the degree of rescue was not as strong as that conferred by activating the Gαs pathway. Furthermore, while the hyperactive locomotion seen in unc-31(e928); gsa-1(ce81) double mutants is highly coordinated, the locomotion of unc-31(e928); egl-30(tg26) double mutants is a mixture of coordinated and uncoordinated movements, with the latter doubles showing a tendency to knot or coil (4.5 ± 0.8% of the time spent in a coiled or knotted state). Interestingly, the egl-30(tg26) gain-of-function mutation has a similar effect on the unc-31 null as it does on a mutant lacking a neuronal Gαs pathway [i.e., the muscle-rescued acy-1(pk1279) mutant]. Both double mutants have similar locomotion rates and show a similar tendency to knot or coil (Figure 5B photo insets and supplemental movies at http://www.genetics.org/supplemental/) (Reynolds et al. 2005). Similarly, activating the downstream priming part of the Gαq pathway by applying phorbol esters to either unc-31 nulls or mutants lacking a neuronal Gαs pathway causes partial suppression of their paralysis, but does not cause hyperactive locomotion (Reynolds et al. 2005) (Table 1). In contrast, phorbol esters induce significantly hyperactive locomotion in an egl-30 (Gαq) reduction-of-function mutant that is substantially more paralyzed than the unc-31 null (Reynolds et al. 2005). Because hyperactivating the Gαq pathway (or adding phorbol esters) has a similar effect on unc-31 null mutants as it does on mutants lacking a neuronal Gαs pathway, these data again support the conclusion that the neuronal Gαs pathway is essentially in an inactive state in unc-31 null mutants. The data do not suggest a specific interaction between UNC-31 and the Gαq pathway since the observed phenotypes are consistent with known interactions between the Gαq and Gαs pathways, and the unc-31 null appears to be behaving as if it lacks a neuronal Gαs pathway.
TABLE 1.
Effects of phorbol esters on the locomotion rate of unc-31 null mutants
| Genotype | Condition | Mean locomotion rate (body bends/min) |
|---|---|---|
| N2 (wild type) | Carrier only | 16.7 ± 2.7 |
| N2 (wild type) | 5 μm phorbol ester | 45.1 ± 1.8 |
| unc-31(e928) | Carrier only | 0.40 ± 0.08 |
| unc-31(e928) | 5 μm phorbol ester | 18.0 ± 1.4 |
Data represent the mean ± standard error of populations of eight animals incubated on plates containing carrier only (0.062% ethanol) or 5 μm phorbol myristate acetate. Animals were plated at staggered intervals and assayed exactly 2.5 hr after contact by counting body bends over a 6-min period.
The unc-31 null mutation has little or no effect on the strong aldicarb hypersensitivity by conditions that activate the Gαq synapse priming pathway:
We next used aldicarb sensitivity assays to investigate the extent to which steady-state acetylcholine release is affected in unc-31 nulls containing activated Gαq or Gαs pathways. Mutants in which the Gαq or Gαs pathways are hyperactivated have normal or even reduced muscle responses to levamisole and yet are hypersensitive to aldicarb (Lackner et al. 1999; Doi and Iwasaki 2002; Schade et al. 2005) (data not shown), which indicates increased levels of steady-state acetylcholine release. As might be expected from their strongly hyperactive locomotion rate, gsa-1(ce81); unc-31(e928) double mutants (Gαs pathway activated + UNC-31 knocked out) were hypersensitive to aldicarb (Figure 5C). Surprisingly, however, the egl-30(tg26); unc-31(e928) double mutant (Gαq pathway activated + UNC-31 knocked out) is strongly hypersensitive to aldicarb, despite its approximately wild-type locomotion rate (Figure 5D). Similarly, both wild-type and unc-31 null mutants in which the downstream priming part of the Gαq pathway has been hyperactivated by phorbol esters are strongly and equally hypersensitive to the paralytic effects of aldicarb (Figure 5E).
GOA-1 (Gαo) negatively regulates the core EGL-30 (Gαq) synapse priming pathway by one or more unknown mechanisms, and knocking out the Gαo pathway causes adult phenotypes similar to those of mutants containing a hyperactivated Gαq pathway (Figure 1) (Hajdu-Cronin et al. 1999; Miller et al. 1999; Nurrish et al. 1999). Despite the fact that goa-1 (Gαo) null single mutants are extremely hypersensitive to aldicarb and exhibit strongly hyperactive locomotion rates, knocking out goa-1 with the goa-1(sa734) null mutation (Robatzek and Thomas 2000) only partially suppresses the paralysis of the unc-31 null, improving its locomotion rate to about half that of wild type (Figure 6B). We obtained similar results in a previous study when the same goa-1 null mutation was moved into a strain lacking a Gαs pathway (Reynolds et al. 2005). This again highlights the similarity between unc-31 null mutants and mutants lacking a neuronal Gαs pathway. Similar to the effect of phorbol esters on unc-31 null mutants, the strong aldicarb hypersensitivity of the goa-1 (Gαo) null was unaffected by the unc-31 null mutation (Figure 6, C and D). In strong contrast, a goa-1 null mutation has little effect on the strong aldicarb resistance and paralysis of the similarly paralyzed egl-30(ad805) (Gαq) strong reduction-of-function mutant (Miller et al. 1999). In other words, similarly paralyzed unc-31 and egl-30 alleles behave opposite to each other when combined with a goa-1 null mutation. These results further support the conclusion that UNC-31 and Gαq function in different pathways and are consistent with our findings in Figure 3C, suggesting that the unc-31 null mutation has little effect on aldicarb sensitivity. The results also show that UNC-31 is not required for the strong aldicarb hypersensitivity caused by hyperactivating the Gαq pathway or knocking out the Gαo pathway.
UNC-31 (CAPS) and the Gαs pathway have a specific functional relationship:
How specific is the interaction between UNC-31 and the Gαs pathway? Can the activated Gαs pathway strongly rescue any similarly paralyzed synaptic signaling mutant? To test this, we asked to what extent activating the Gαs pathway rescues egl-30 (Gαq) reduction of function mutants that are just as paralyzed as the unc-31 null. We found that activating various parts of the Gαs pathway by any one of three different mutations caused an ∼50-fold improvement in the locomotion rate of the unc-31 null (Figure 7A). In contrast, these same mutations only weakly improve the locomotion rate of the egl-30(ad805) reduction-of-function mutant, and the egl-30(md186) reduction-of-function mutant is improved only fivefold, even though it is approximately six times more active than the unc-31 null as a single mutant (Figure 7A). These results again point to a specific functional relationship between UNC-31 and the Gαs pathway.
The activated Gαs pathway can rescue the paralysis of adults lacking UNC-31 by rapid, functional changes:
Is the activated Gαs pathway's ability to rescue the paralysis of unc-31 null mutants a consequence of altered neuronal development or is it caused by functional changes in fully developed neurons? To test this, we transferred the unc-31 null mutation into a background containing the gsa-1 Q208L gain-of-function transgene under control of a heat-shock-inducible promoter, which was produced in a previous study (Korswagen et al. 1997). As shown in Figure 7B, neither wild type nor the unc-31 null single mutant shows an altered locomotion rate after heat shock and recovery. In the absence of heat-shock treatment, the locomotion rate of the unc-31 null containing the gsa-1 gain-of-function transgene is improved severalfold over the unc-31 null single mutant, but is still approximately sevenfold less active than wild type. This is likely due to a small amount of expression of the highly potent gsa-1 gain-of-function transgene from the uninduced heat shock promoter. In contrast, the unc-31 null containing the gsa-1 gain-of-function transgene is strongly rescued, to hyperactive levels of locomotion more than double that of wild type, only 3 hr after the transgene is turned on by a 40-min heat-shock treatment of adult animals. We conclude that both the paralysis of unc-31 null mutants and the rescue of unc-31 nulls caused by activating the Gαs pathway are the result of functional (short timescale) defects/events. However, we note that this timescale does not exclude structural changes in synapses or in populations of synapses.
The neuronal, and not the muscle, Gαs pathway contributes most significantly to rescuing the paralysis of the unc-31 null:
The C. elegans Gαs pathway functions in both neurons and muscles (Reynolds et al. 2005). Which Gαs pathway is responsible for rescuing unc-31's paralysis? To test this, we analyzed strains carrying the unc-31 null mutation in combination with transgenic mutations that activate either the neuronal Gαs pathway or the muscle Gαs pathway. For this purpose we used the ceIs6 and ceIs11 transgenes that specifically hyperactivate the Gαs pathway in the nervous system or body wall muscles, respectively, via the ACY-1 gain-of-function mutation P260S (Schade et al. 2005). We found that the locomotion rate of the unc-31 null is greatly improved by the native ce2 mutation, which activates ACY-1 both in neurons and in muscle; however, nearly the same level of rescue is caused by a transgene expressing the same mutation only in the nervous system (Figure 8A). In contrast, expressing the same mutation only in muscle does not affect even the paralysis of the unc-31 null (Figure 8A). The integrated muscle-driven transgene is likely to be fully functional because it causes strongly hyperactive locomotion and significant hypersensitivity to the acetylcholine receptor agonist levamisole (Schade et al. 2005). These results suggest that it is activation of the neuronal Gαs pathway that contributes most significantly to rescuing the paralysis of the unc-31 null. Although the results do not rule out a contribution from the muscle Gαs pathway, they do suggest that the muscle Gαs pathway cannot unilaterally rescue the unc-31 null, as can the neuronal Gαs pathway.
Figure 8.
Transgenic site-of-action studies using cell-specific promoters. (A) The neuronal, and not the muscle, Gαs pathway contributes most significantly to rescuing the paralysis of the unc-31 null. Shown are the mean locomotion rates, expressed as body bends per minute, of various strains. The first group of four bars on the left represents wild-type and single-mutant control strains, which are color coded in the inset. In the right set of bars, double- and single-mutant strains homozygous for unc-31(e928) are grouped together as indicated and labeled. Gαs pathway activation transgenes consisted of an acy-1 cDNA containing the P260S gain-of-function mutation (Reynolds et al. 2005) driven by either the rab-3 or the myo-3 promoter (for neuronal or muscle-specific expression, respectively). acy-1(ce2) is the native genomic mutation containing the P260S gain-of-function mutation. Error bars represent the standard error of the mean for 10 animals. The values for the acy-1(ce2), ceIs11, and ceIs6 single mutants are reprinted with permission from Schade et al. (2005) for comparison. (B) UNC-31 and the neuronal Gαs pathway can function together in the same neurons. Shown are the mean locomotion rates, expressed as body bends per minute, of various strains. Note that the unc-17β promoter used here does not drive expression in the VC neurons in the ventral cord, which could also contribute to locomotion rate. Error bars represent the standard error of the mean for 10 animals. Transgenic array descriptions are ceEx117 [rab-3∷unc-31(+) cDNA], ceIs19 [unc-17β∷unc-31(+) cDNA], and ceIs28 [unc-17β∷acy-1(P260S gain-of-function) cDNA].
UNC-31 and the Gαs pathway can function in the same cells to regulate locomotion:
We next used cell-specific promoters to investigate where within the nervous system unc-31 acts. We found that expressing the unc-31 cDNA throughout the nervous system restores a wild-type locomotion rate to the unc-31 null (Figure 8B). The ventral cord cholinergic neurons provide the major excitatory input for regulating locomotion rate in C. elegans. We used the cholinergic-specific promoter unc-17β to test whether or not UNC-31 exerts its effects on locomotion rate from the ventral cord cholinergic neurons. The unc-17β promoter lacks promoter elements necessary for expression in head cholinergic neurons and drives expression only in the ventral cord cholinergic neurons, with the exception of the hermaphrodite-specific VC neurons (J. Rand, personal communication). Using this promoter, we found that expressing the unc-31 cDNA just in the ventral cord cholinergic motor neurons also restores a near-wild-type locomotion rate to the unc-31 null (∼80% of wild type; Figure 8B). This result suggests that the paralysis of unc-31 nulls can largely be accounted for by lack of UNC-31 in the ventral cord cholinergic neurons. Since UNC-31 functions upstream of the Gαs pathway and since the ventral cord cholinergic neurons make synapses onto muscle cells, these data suggest that both UNC-31 and the neuronal Gαs pathway function in the ventral cord cholinergic neurons to regulate locomotion rate. To test this inference, we produced a transgenic strain containing the acy-1 (P260S) Gαs pathway-activating mutation (a moderate activator of the Gαs pathway) (Reynolds et al. 2005) under control of the unc-17β promoter. When we integrated this transgene and transferred it into the unc-31 null background, we found that the transgene significantly rescued the paralysis of the unc-31 null, improving its locomotion rate to a level that is ∼50% of the wild-type rate (Figure 8B). This suggests that UNC-31 and the Gαs pathway can function in the same neurons to regulate locomotion rate, and those neurons prominently include the ventral cord cholinergic motor neurons.
In nerve cords UNC-31 is concentrated largely at cholinergic motor neuron synapses:
To further investigate where UNC-31 acts, we raised an antibody to the UNC-31 protein and used it to immunostain whole animals. Our antibody is completely specific for the UNC-31 protein because the staining is absent in the unc-31(e928) null deletion mutant (supplemental Figure 1 at http://www.genetics.org/supplemental/). Consistent with our transgenic site-of-action studies, we found that, within the ventral, dorsal, and sublateral nerve cords, most of the concentrated areas of UNC-31 are located in cholinergic synapses, which we identified by costaining with an antibody to the synaptic vesicle ACh transporter UNC-17 (Figures 9A and 10A). To determine if UNC-31 localizes to any noncholinergic synapses in the nerve cords we included a third antibody to the pan-synaptic protein UNC-10 (RIM) in the staining reaction (Koushika et al. 2001). Within the ventral and dorsal nerve cords, we observed only a few examples of concentrated UNC-31 colocalizing with noncholinergic UNC-10 spots (Figure 10B). An unexpected conclusion of these findings is that UNC-31 is not concentrated at most noncholinergic synapses in the nerve cords, such as the GABAergic, glutamatergic, and catecholaminergic synapses. However, within the nerve ring, which contains a large number of interneuronal synapses in the animal's head, UNC-31 is clearly concentrated in regions containing large numbers of noncholinergic synapses (data not shown).
Figure 9.
Within the ventral nerve cord, UNC-31 is concentrated presynaptically at or near active zones and largely at cholinergic synapses. (A) UNC-31 immunostaining in the ventral nerve cord. Maximum intensity projections of a z-series through the cord are shown. The four panels are different color combinations of the same image. (Top) Red channel showing UNC-17 staining to mark cholinergic vesicle clusters. (Second channel) Merged UNC-17 (red) and UNC-31 (green) channels showing that UNC-31 is concentrated within cholinergic synapses in the ventral cord. (Third channel) Locations of active zones at cholinergic synapses as indicated by an antibody to UNC-10 (blue). Most of the blue spots not associated with cholinergic synapses likely indicate the locations of noncholinergic synapses in the cord. (Bottom) Merged image of all three channels. (B) Enlargements of clusters of synapses in the ventral cord showing that UNC-31 and UNC-10 often localize to the same subsynaptic region (at or near the active zone). Asterisks indicate synaptic sites having strong UNC-31 staining, but weak or little UNC-10. Arrowheads indicate a few examples of synapses with strong UNC-10 and weak UNC-31. Arrows in the bottom right panel indicate noncholinergic active zones that lack concentrated UNC-31.
Figure 10.
UNC-31 immunolocalization in the dorsal and sublateral nerve cords. (A) UNC-31 immunostaining in the dorsal nerve cord. Maximum intensity projections of a z-series through the cord are shown. The three panels are different color combinations of the same image. (Top) Locations of active zones (blue UNC-10) at cholinergic synapses (red UNC-17). Most of the blue spots not associated with cholinergic synapses likely indicate the locations of noncholinergic synapses in the cord (e.g., GABAergic, glutamatergic, and catacholaminergic synapses). (Middle) Merged UNC-17 (red) and UNC-31 (green) channels showing that UNC-31 is largely concentrated within cholinergic synapses in the ventral cord. Note the high correlation of UNC-31 spots with active zones shown in the top channel. (Bottom) Merged image of all three channels. White spots indicate colocalization of all three markers. Arrows at the far left indicate the likely locations of two noncholinergic synapses with significant UNC-31 staining. (B) Enlargement of cholingergic synapses in one of the sublateral nerve cords showing that UNC-31 and UNC-10 often localize to the same subsynaptic region (the active zone). A small synapse containing concentrated UNC-31, but little UNC-10 is indicated with an arrow.
UNC-31 is often concentrated at or near active zones:
At many cholinergic synapses in the nerve cords, UNC-31 is clearly concentrated at one or a few subsynaptic regions. To determine the subregion to which UNC-31 localizes, we compared the locations of the UNC-31 spots to the locations of UNC-10 (RIM). UNC-10 and its vertebrate ortholog RIM localize to the active zone of synapses (Wang et al. 1997; Koushika et al. 2001). This is the region where neurotransmitter is most likely to be released and is defined by docked vesicles in electron micrographs. At the light microscope level, UNC-10 spots cover a larger area than the size of active zones, probably due to the limits of light-level resolution. We found that UNC-31 is often highly concentrated in subsynaptic regions that overlap precisely with UNC-10 at the theoretical limit of light microscope resolution (Figures 9, A and B, and 10, A and B). Although most of the UNC-31 spots correspond to UNC-10 active zones, our analysis revealed three prominent classes of active zones based on widely differing ratios of UNC-31 and UNC-10 at active zones. One class is enriched for UNC-31 but has extremely low amounts of UNC-10. A second class is enriched for UNC-10 but has very low amounts of UNC-31. The third class, composed of all other active zones, has less divergent UNC-10/UNC-31 intensity ratios. Readers can view examples of all three classes in the enlargements in Figures 9B and 10B, which depict ventral and sublateral nerve cord synapses, respectively, from different adult animals. We quantified the UNC-31/UNC-10 intensity ratios over a 50-μm region of the ventral nerve cord (∼5% of the animal's length) containing 101 cholinergic vesicle clusters. We found that ∼12% of UNC-31 spots at synapses have >10-fold lower intensities of UNC-10 (ratios ranged from 10 to 520), while another 7% have only 4- to 9-fold lower intensities of UNC-10. Conversely, ∼9% of UNC-10 spots have 10- to 46-fold lower UNC-31 intensities and another 13% have only 4- to 8-fold lower UNC-31 intensities. The remaining ∼59% of the regions we analyzed had UNC-31/UNC-10 intensity ratios that differ by <4-fold (supplemental Tables 1 and 2 at http://www.genetics.org/supplemental/). These data do not address whether the enrichment or exclusion of UNC-31 and UNC-10 at specific active zones in adult animals is a dynamic process or an unchanging characteristic of mature synapses.
Another notable feature of UNC-31 staining in the nerve cords is that it often differs dramatically between different synapses. Some synapses are diffusely but almost entirely filled with UNC-31, with focal concentrations at UNC-10 positive regions and sometimes at other regions as well [e.g., see Figures 9B (top right) and 10A]. In contrast, other synapses have only one or more tightly localized UNC-31 foci, at least one of which often corresponds to an UNC-10 spot (Figures 9 and 10).
DISCUSSION
Our interest in this study began with the striking observation, reported earlier, that animals lacking a neuronal Gαs pathway are strongly paralyzed despite being devoid of permanent developmental defects and despite exhibiting normal levels of steady-state acetylcholine release as measured by aldicarb sensitivity. To further investigate the nature of the puzzling neuronal Gαs pathway, we took a genetic approach to look for mutants sharing a similar spectrum of phenotypes. Our genetic investigation of a mutant lacking the CAPS dense-core vesicle priming protein led to the unexpected findings that CAPS is specifically required to activate the neuronal Gαs pathway, that both CAPS and neuronal Gαs can function from the same motor neurons to regulate locomotion, and that CAPS is often concentrated at or near active zones of motor neuron synapses.
Presynaptic UNC-31 (CAPS) is required to activate the neuronal Gαs pathway:
As we show in this study, mutants lacking the sole C. elegans ortholog of CAPS share a similar unusual spectrum of phenotypes with mutants lacking a neuronal Gαs pathway, including strong paralysis combined with near-normal steady-state acetylcholine release, as measured by drug sensitivity assays. A Drosophila study also reported severe sluggishness and normal spontaneous neurotransmitter release in a mutant lacking CAPS; however, evoked release was decreased by only ∼50% (Renden et al. 2001). A decrease of that relatively small magnitude could explain the moderate aldicarb resistance that we observed in the unc-31 null in multiple independent trials at low and medium concentrations of aldicarb. A previous study found only one unc-31 allele in a screen for aldicarb-resistant mutants that produced 29 alleles of the similarly paralyzed, similar-sized unc-13 gene (Miller et al. 1996). Our finding that the unc-31 null is indistinguishable from wild type with respect to the aldicarb concentration required to achieve zero growth likely accounts for unc-31's low representation in that screen. An investigation of mice lacking CAPS-1 failed to find any synaptic transmission phenotype in several different brain regions where CAPS-1 is thought to play an important role, although that study could not explicitly rule out the contributions of CAPS-2 in those brain regions (Speidel et al. 2005).
We used genetic pathway analysis to investigate whether or not the shared phenotypes of unc-31 and Gαs pathway null mutants reflect action in the same pathway. Our analysis revealed that UNC-31 and Gαs are indeed different parts of the same pathway for regulating locomotion rate, because knocking out unc-31, or the Gαs pathway, or both simultaneously, results in similar locomotion rates (∼2–3% of wild type). Using epistasis analysis we then showed that, within the UNC-31/Gαs pathway, UNC-31 is the upstream component because the unc-31 null mutation has almost no effect on the strongly hyperactive locomotion rate of mutants with a hyperactivated Gαs pathway.
The UNC-31/Gαs pathway is functionally distinct from the Gαq pathway:
We also showed that strongly activating the EGL-30 (Gαq) pathway improves the locomotion rate of the unc-31 null to about wild-type levels. From this result alone, one cannot rule out the possibility that UNC-31 also plays an upstream role in activating the Gαq pathway. However, we showed that the locomotion phenotype of double mutants containing knocked out UNC-31 + activated Gαq is similar to that of double mutants containing no neuronal Gαs pathway + activated Gαq. Therefore, explaining this result does not require invoking a specific interaction between UNC-31 and the Gαq pathway, since the unc-31 null is behaving simply like a mutant lacking a neuronal Gαs pathway. Three other lines of evidence presented in this article suggest that presynaptic UNC-31 specifically interacts with the Gαs pathway and does not directly interact with the Gαq pathway. First, the aldicarb sensitivity of an unc-31 null mutant resembles a neuronal Gαs pathway null and differs widely from a similarly paralyzed egl-30 mutant. Second, knocking out unc-31 in a strain that has no Gαs pathway has no further effect on locomotion rate (even though 2–3% of locomotion rate function remains in each strain). If the unc-31 null mutation also significantly reduced activity of the Gαq pathway, then knocking out unc-31 in a strain that has no Gαs pathway should worsen the locomotion rate because both the Gαq and the Gαs pathways would be affected. Third, similarly paralyzed unc-31 and egl-30 alleles behave opposite to each other, with respect to aldicarb sensitivity, when combined with a goa-1 null mutation (this study; Miller et al. 1999). Together, these findings suggest that the UNC-31/Gαs pathway is functionally distinct from the Gαq pathway with which it interacts as part of the synaptic signaling network (Reynolds et al. 2005). Consistent with these findings, the strong synthetic interaction we observed when we reduced egl-30 (Gαq) function in the unc-31 null mutant background is most likely caused by simultaneous disruption of the Gαq pathway (through the egl-30 reduction-of-function mutations) and functional elimination of the Gαs pathway (through the unc-31 null mutation).
UNC-31 (CAPS) and Gαs function in the same neurons to regulate locomotion rate:
Our transgenic site-of-action studies show that UNC-31 and the Gαs pathway can function in the same neurons to regulate locomotion rate and that those neurons prominently include the ventral cord cholinergic motor neurons. This was an unexpected finding, because a Drosophila study found that transgenic CAPS expression in motor neurons (where the native CAPS protein is also robustly expressed) is not sufficient to rescue, or even affect, the ∼50% decrease in glutamatergic synaptic transmission at motor neuron synapses (Renden et al. 2001). From that result, the authors logically concluded that the role of CAPS in neuromuscular glutamate synaptic transmission is secondary to CAPS function in a different cell. Although species-specific differences in synaptic architecture could account for these very different results, we also note that the two studies used different outputs to test rescue. Our study focused primarily on the rescue of unc-31's paralysis and used locomotion rate as the output, whereas the Drosophila study focused on whether or not the 50% decrease in synaptic transmission was rescued and did not report effects on locomotion. It remains possible that the relatively modest 50% decrease in synaptic transmission in Drosophila CAPS mutants is only partially related or unrelated to the reported strong sluggishness of these mutants (Renden et al. 2001). We also note that the Drosophila study did not demonstrate CAPS rescue in a nonmotor neuron subset of neurons, only absence of rescue by a promoter driving expression in a small subset of the total motor neurons. A role of some kind for Drosophila CAPS in motor neurons would not be surprising given the robust expression of the native CAPS protein in motor neurons (Renden et al. 2001).
Our immunolocalization studies showed that, within the nerve cords, UNC-31 is especially concentrated in the cholinergic synapses implicated by our transgenic site-of-action studies; however, we are not claiming that UNC-31 is localized only to cholinergic synapses in C. elegans. As noted, we identified some concentrated UNC-31 spots in the nerve cords that colocalized with noncholinergic UNC-10 spots as well as strong UNC-31 staining in the nerve ring, only part of which overlaps with our marker for cholinergic synapses. UNC-31's ability to drive locomotion from the cholinergic motor neuron synapses and the UNC-31 protein's disproportionate concentration at cholinergic synapses in the nerve cords (as opposed to the GABAergic, glutamatergic, and catecholaminergic synapses also present in the nerve cords) may reflect a specific and critical role for UNC-31 at cholinergic motor neuron synapses. Although transmitter-specific synaptic variation in CAPS-1 staining has been reported in mouse, it is not known whether or not the synapses lacking concentrated CAPS-1 contain the CAPS-2 isoform (Speidel et al. 2003).
UNC-31/RIM ratios define specific classes of active zones:
In this study we showed for the first time that presynaptic UNC-31 can be highly concentrated at or near active zones. Recent studies have shown that vertebrate CAPS-1 activity and binding to the plasma membrane of PC12 cells depend on the lipid PIP2 (Grishanin et al. 2004), which is concentrated in submicrometer-size microdomains that correlate with sites of dense-core vesicle exocytosis in PC12 and chromaffin cells (Aoyagi et al. 2005; Milosevic et al. 2005). Further studies will be necessary to determine whether or not UNC-31's recruitment to high focal concentrations at active zones is PIP2 dependent. We also showed that the ratio of UNC-31 to the active zone marker UNC-10 (RIM) can differ markedly between different active zones. Future studies could address whether the enrichment or exclusion of UNC-31 at synaptic active zones in adult animals is a dynamic process (controlled by signals of unknown origin) or a static characteristic of mature synapses.
The UNC-31/neuronal Gαs pathway:
Our findings suggest that UNC-31 (CAPS) and neuronal Gαs form a pathway with UNC-31 as the required upstream activator and that both components exert their effects from the same motor neurons to regulate locomotion rate. The simplest model that is consistent with these data, and with what we know from biochemical studies of CAPS, is that UNC-31 exerts its effects on the Gαs pathway via local exocytosis of dense-core vesicles. Figure 11 depicts two possible mechanisms. As indicated by the results in this article, Figure 11, A and B, shows UNC-31 acting in the same cells as neuronal Gαs, in the same molecular pathway as Gαs, upstream of Gαs, with a site of action at or near the synaptic active zone, and not directly interacting with the Gαq pathway. Figure 11A shows UNC-31 exerting its effects on the Gαs pathway via exocytosis of a dense-core vesicle neurotransmitter/neuropeptide, which then locally activates a G protein-coupled receptor that is already present in the presynaptic membrane. Figure 11B shows UNC-31 exerting its effects on the Gαs pathway via exocytotic insertion of one or more G protein-coupled receptors in the presynaptic membrane, similar to the phenomena recently described in vertebrate neuronal cell bodies (Guan et al. 2005). An event such as this would allow the Gαs pathway to be locally activated (at specific synapses) in response to outside signals (which could be either local or nonlocal in nature). In a third model (not shown in Figure 11) UNC-31 (CAPS) exerts its effects on the Gαs pathway entirely via intracellular interactions (direct or indirect, but not via dense-core vesicle exocytosis). Significant variations of these models are not excluded by the data. Even if CAPS activates the Gαs pathway via secretion of a neurotransmitter or neuropeptide from dense-core vesicles, we cannot exclude an indirect action via an intermediate cell as opposed to a direct autocrine action or local receptor insertion.
Figure 11.
Alternative models for the function of presynaptic UNC-31 (CAPS). As indicated by our results, both A and B show UNC-31 acting in the same cells as neuronal Gαs, in the same molecular pathway as Gαs, upstream of Gαs, with a site of action at or near the synaptic active zone, and not directly interacting with the Gαq pathway. Both models depict CAPS acting as a dense-core vesicle priming protein as described in vertebrate studies (Grishanin et al. 2004). (A) UNC-31 exerts its effects on the Gαs pathway via exocytosis of a dense-core vesicle neurotransmitter/neuropeptide that then locally activates a G protein-coupled receptor that is already present in the presynaptic membrane. (B) UNC-31 exerts its effects on the Gαs pathway via exocytotic deposition of one or more G protein-coupled receptors in the presynaptic membrane, similar to the phenomena described in vertebrate neuronal cell bodies (Guan et al. 2005).
We also note that our data do not address the relationship between presynaptic UNC-31 and neuropeptide secretion; however, we note that animals lacking the neuropeptide processing enzymes EGL-3 (prohormone convertase 2) or EGL-21 (carboxypeptidase) do not closely phenocopy unc-31 null mutants. For example, egl-3 null mutants are approximately six times more active than unc-31 null mutants by a locomotion rate assay (K.G.M., data not shown) and yet, unlike unc-31 null mutants, both egl-3 and egl-21 single and double mutants exhibit a significant aldicarb resistance phenotype (Jacob and Kaplan 2003). These observations seem to suggest that the function of UNC-31 is not limited to mediating the secretion of neuropeptides and may include functions distinct from those of neuropeptides, such as (for example) the regulated insertion of presynaptic Gαs-coupled receptors, as depicted in Figure 11B. UNC-31 may also mediate the secretion of larger signaling proteins (analogous to vertebrate BDNF, for example) and/or the receptors for such proteins. These larger signaling proteins may not require processing by EGL-3 or EGL-21, thus explaining the phenotypic differences that distinguish unc-31 from egl-3/egl-21 mutants.
Conclusion:
Our data suggest that presynaptic UNC-31 activity, possibly acting via dense-core vesicle exocytosis, is required to locally activate the neuronal Gαs pathway near synaptic active zones. Whether the main mediator of UNC-31's action is a secreted molecule or a receptor inserted by regulated exocytosis is the subject of alternative hypotheses. Signaling molecules regulating the highly focal localization of UNC-31 may provide a spatial or positional mechanism to control local activation of the neuronal Gαs pathway during the execution of behaviors. Future studies will be required to determine whether or not there are signal transduction proteins upstream of UNC-31 that control activation of the UNC-31–Gαs pathway.
Acknowledgments
The authors thank Celine Moorman and Ron Plasterk for the acy-1(pk1279) allele, Kouichi Iwasaki for providing his egl-30(tg26) mutant ahead of publication, Jim Thomas for goa-1(sa734), and Leon Avery for providing the unc-31(e928) mutant. We also thank Janet Duerr and Jim Rand for their gift of the UNC-17 antibody and Mike Nonet for his gift of the UNC-10 antibody. Jim Rand also provided the unc-17β promoter plasmid and insights regarding the alternative models presented in the discussion. All DNA sequences were obtained from the Oklahoma Medical Research Foundation's Core DNA Sequencing Facility. Some of the strains used here were provided by the C. elegans Genetics Center. Grants to K.G.M. from the National Institute of Mental Health (MH62400) and from the Oklahoma Center for the Advancement of Science and Technology (HR02-093) supported this work.
References
- Aoyagi, K., T. Sugaya, M. Umeda, S. Yamamoto, S. Terakawa et al., 2005. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 280: 17346–17352. [DOI] [PubMed] [Google Scholar]
- Aravamudan, B., T. Fergestad, W. S. Davis, C. K. Rodesch and K. Broadie, 1999. Drosophila Unc-13 is essential for synaptic transmission. Nat. Neurosci. 2(11): 965–971. [DOI] [PubMed] [Google Scholar]
- Augustin, I., C. Rosenmund, T. C. Südhof and N. Brose, 1999. Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400: 457–461. [DOI] [PubMed] [Google Scholar]
- Avery, L., C. I. Bargmann and H. R. Horvitz, 1993. The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics 134: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, A. J., A. C. Hart and J. M. Kaplan, 1998. Gαs-induced neurodegeneration in Caenorhabditis elegans. J. Neurosci. 18: 2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage, L., L. Avery, A. Katz, U. Kim, J. E. Mendel et al., 1996. Mutations in a C. elegans Gqα gene disrupt movement, egg laying, and viability. Neuron 16: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, D. L., G. Patikoglou and M. R. Koelle, 2001. Two RGS proteins that inhibit Gαo and Gαq signaling in C. elegans neurons require a Gβ5-like subunit for function. Curr. Biol. 11: 222–231. [DOI] [PubMed] [Google Scholar]
- Chen, C., and W. G. Regehr, 1997. The mechanism of cAMP-mediated enhancement at a cerebellar synapse. J. Neurosci. 17: 8687–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, M., and K. Iwasaki, 2002. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron 33: 249–259. [DOI] [PubMed] [Google Scholar]
- Duerr, J. S., J. Gaskin and J. B. Rand, 2001. Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoamines. Am. J. Physiol. Cell. Physiol. 280: C1616–C1622. [DOI] [PubMed] [Google Scholar]
- Grishanin, R. N., V. A. Klenchin, K. M. Loyet, J. A. Kowalchyk, K. Ann et al., 2002. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J. Biol. Chem. 277: 22025–22034. [DOI] [PubMed] [Google Scholar]
- Grishanin, R. N., J. A. Kowalchyk, V. A. Klenchin, K. Ann, C. A. Earles et al., 2004. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron 43: 551–562. [DOI] [PubMed] [Google Scholar]
- Guan, J. S., Z. Z. Xu, H. Gao, S. Q. He, G. Q. Ma et al., 2005. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell 122: 619–631. [DOI] [PubMed] [Google Scholar]
- Hajdu-Cronin, Y. M., W. J. Chen, G. Patikoglou, M. R. Koelle and P. W. Sternberg, 1999. Antagonism between Goα and Gqα in C. elegans: the RGS protein EAT-16 is necessary for Goα signaling and regulates Gqα activity. Genes Dev. 13: 1780–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J. C., and T. F. Martin, 1992. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J. Cell Biol. 119: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, D., K. Suzuki, W. J. Wolfgang, C. Clay, M. Forte et al., 2003. Presynaptic impairment of synaptic transmission in Drosophila embryos lacking Gsα. J. Neurosci. 23: 5897–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, T. C., and J. M. Kaplan, 2003. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23: 2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro, Y., H. Kuromi, R. Delgado, C. Maureira, C. Oliva et al., 2004. Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res. Brain Res. Rev. 47: 18–32. [DOI] [PubMed] [Google Scholar]
- Koelle, M. R., and H. R. Horvitz, 1996. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 84: 112–125. [DOI] [PubMed] [Google Scholar]
- Korswagen, H. C., J. Park, Y. Ohshima and R. H. Plasterk, 1997. An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev. 11: 1493–1503. [DOI] [PubMed] [Google Scholar]
- Koushika, S. P., J. E. Richmond, G. Hadwiger, R. M. Weimer, E. M. Jorgensen et al., 2001. A post-docking role for active zone protein Rim. Nat. Neurosci. 4: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner, M. R., S. J. Nurrish and J. M. Kaplan, 1999. Facilitation of synaptic transmission by EGL-30 Gqα and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346. [DOI] [PubMed] [Google Scholar]
- Livingstone, D., 1991. Studies on the unc-31 gene of Caenorhabditis elegans. Ph.D. Thesis, University of Cambridge, Cambridge, UK.
- Martin, T. F., 2003. Tuning exocytosis for speed: fast and slow modes. Biochim. Biophys. Acta 1641: 157–165. [DOI] [PubMed] [Google Scholar]
- Maruyama, I. N., and S. Brenner, 1991. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 88: 5729–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10(12): 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel, J. E., H. C. Korswagen, K. S. Liu, Y. M. Hajdu-Cronin, M. I. Simon et al., 1995. Participation of the protein Go in multiple aspects of behavior in C. elegans. Nature 267: 1652–1655. [DOI] [PubMed] [Google Scholar]
- Miller, K. G., A. Alfonso, M. Nguyen, J. A. Crowell, C. D. Johnson et al., 1996. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA 93: 12593–12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. G., M. D. Emerson and J. B. Rand, 1999. Goα and diacylglycerol kinase negatively regulate the Gqα pathway in C. elegans. Neuron 24: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. G., M. D. Emerson, J. McManus and J. B. Rand, 2000. RIC-8 (synembryn): a novel conserved protein that is required for Gqα signaling in the C. elegans nervous system. Neuron 27: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic, I., J. B. Sorensen, T. Lang, M. Krauss, G. Nagy et al., 2005. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 25: 2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman, C., and R. H. Plasterk, 2002. Functional characterization of the adenylyl cyclase gene sgs-1 by analysis of a mutational spectrum in Caenorhabditis elegans. Genetics 161: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natochin, M., and N. O. Artemyev, 2003. A point mutation uncouples transducin-alpha from the photoreceptor RGS and effector proteins. J. Neurochem. 87: 1262–1271. [DOI] [PubMed] [Google Scholar]
- Nurrish, S., L. Segalat and J. M. Kaplan, 1999. Serotonin inhibition of synaptic transmission: Gαo decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242. [DOI] [PubMed] [Google Scholar]
- Rand, J. B., and M. L. Nonet, 1997. Synaptic transmission, pp. 611–643 in C. elegans II, edited by D. H. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Renden, R. B., and K. Broadie, 2003. Mutation and activation of Gαs similarly alters pre- and postsynaptic mechanisms modulating neurotransmission. J. Neurophysiol. 89: 2620–2638. [DOI] [PubMed] [Google Scholar]
- Renden, R., B. Berwin, W. Davis, K. Ann, C. T. Chin et al., 2001. Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron 31: 421–437. [DOI] [PubMed] [Google Scholar]
- Reynolds, N. K., M. A. Schade and K. G. Miller, 2005. Convergent, RIC-8 dependent Gα signaling pathways in the C. elegans synaptic signaling network. Genetics 169: 650–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond, J. E., W. S. Davis and E. M. Jorgensen, 1999. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci. 2(11): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond, J. E., R. M. Weimer and E. M. Jorgensen, 2001. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 412: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek, M., and J. H. Thomas, 2000. Calcium/calmodulin-dependent protein kinase II regulates Caenorhabditis elegans locomotion in concert with a Go/Gq signaling network. Genetics 156: 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek, M., T. Niacaris, K. Steger, L. Avery and J. H. Thomas, 2001. eat-11 encodes GPB-2, a Gβ5 ortholog that interacts with Goα and Gqα to regulate C. elegans behavior. Curr. Biol. 11: 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade, M. A., N. K. Reynolds, C. M. Dollins and K. G. Miller, 2005. Mutations that rescue the paralysis of C. elegans ric-8 (Synembryn) mutants activate the Gαs pathway and define a third major branch of the synaptic signaling network. Genetics 169: 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalat, L., D. A. Elkes and J. M. Kaplan, 1995. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Nature 267: 1648–1651. [DOI] [PubMed] [Google Scholar]
- Speidel, D., F. Varoqueaux, C. Enk, M. Nojiri, R. N. Grishanin et al., 2003. A family of Ca2+-dependent activator proteins for secretion: comparative analysis of structure, expression, localization, and function. J. Biol. Chem. 278: 52802–52809. [DOI] [PubMed] [Google Scholar]
- Speidel, D., C. E. Bruederle, C. Enk, T. Voets, F. Varoqueaux et al., 2005. CAPS1 regulates catecholamine loading of large dense-core vesicles. Neuron 46: 75–88. [DOI] [PubMed] [Google Scholar]
- Stevens, C. F., and J. M. Sullivan, 1998. Regulation of the readily releasable vesicle pool by protein kinase C. Neuron 21: 885–893. [DOI] [PubMed] [Google Scholar]
- Tall, G. G., A. M. Krumins and A. G. Gilman, 2003. Mammalian Ric-8A (Synembryn) is a heterotrimeric G alpha protein guanine nucleotide exchange factor. J. Biol. Chem. 278: 8356–8362. [DOI] [PubMed] [Google Scholar]
- van der Linden, A. M., F. Simmer, E. Cuppen and R. H. Plasterk, 2001. The G-protein β-subunit GPB-2 in Caenorhabditis elegans regulates the Goα-Gqα signaling network through interactions with the regulator of G-protein signaling proteins EGL-10 and EAT-16. Genetics 158: 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani, T., M. Ertunc, Y. Sara, M. Mozhayeva and E. T. Kavalali, 2005. Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling. J. Neurosci. 25: 10922–10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., M. Okamoto, F. Schmitz, K. Hofmann and T. C. Sudhof, 1997. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 388: 593–598. [DOI] [PubMed] [Google Scholar]
- Waters, J., and S. J. Smith, 2000. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J. Neurosci. 20: 7863–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]