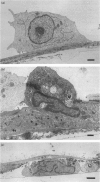
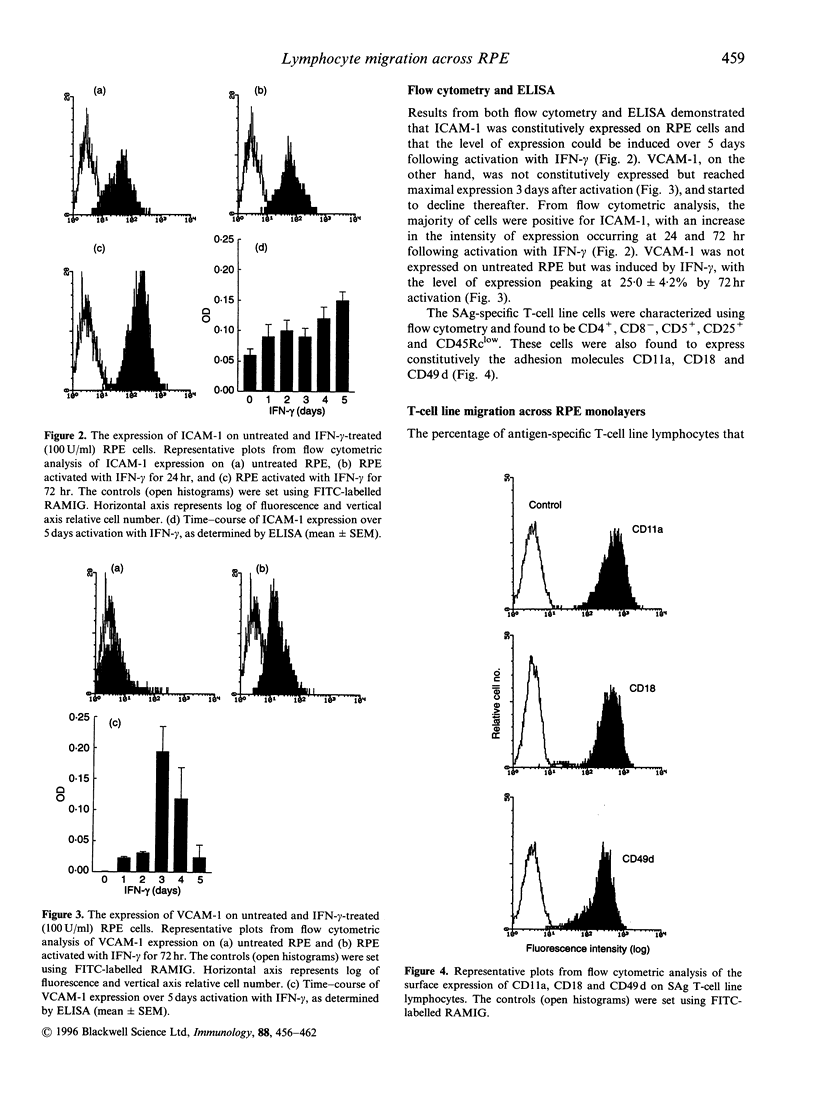
Abstract
The blood-retinal barrier (BRB), which is composed of the retinal pigment epithelium (RPE) and retinal vascular endothelium, normally restricts the traffic of lymphocytes into the retina. During ocular inflammatory conditions such as posterior uveitis there is a large increase in lymphocyte migration across the BRB. The differential role played by the two barrier sites, however, remains unclear. To evaluate the role of the posterior BRB, the migration of CD4+ antigen-specific T-cell line through rat RPE cell monolayers was investigated in vitro using time-lapse videomicroscopy. The adhesion molecules involved in controlling transepithelial migration across normal and interferon-gamma (IFN-gamma)-activated RPE was assessed with monoclonal antibodies directed against cell adhesion molecules. Lymphocytes were treated with antibodies specific for CD11a (alpha L subunit of LFA-1), CD18 (beta 2 subnit of the leucam family) and CD49 d (alpha 4 subnit of very late activation antigen-4, VLA-4), and the RPE with antibodies specific for CD54 (intracellular adhesion molecule-1, ICAM-1) and CD 106 (vascular cell adhesion molecule-1, VCAM-1). Migration across unstimulated RPE was inhibited by antibodies to ICAM-1 (48.6 +/- 3.5% reduction), leucocyte functional antigen-1 (LFA-1) alpha (61 +/- 5.2%) and LFA-1 beta (63.2 +/- 4.7%), but not by antibodies to VLA-4. VCAM-1 was not expressed on untreated RPE. Following activation of the RPE monolayers for 72 hr with IFN-gamma, antibodies to LFA-1 alpha, LFA-1 beta and ICAM-1 inhibited migration by 49.9 +/- 9.4%, 63.6 +/- 5.5% and 47.7 +/- 4.2% respectively. Antibodies to VLA-4 and VCAM-1 blocked migration by 21.5 +/- 8.4% and 32.3 +/- 6.2%, respectively, which correlated with the induction of VCAM-1 expression on RPE and increased migration. Under these conditions blocking both VCAM-1 and ICAM-1 reduced migration by 70.9 +/- 2.3%, which was greater than the effect of blocking either of these molecules alone. These results demonstrate that the posterior barrier of the BRB utilizes the same principle receptor-ligand pairings in controlling lymphocyte traffic into the retina as the vascular endothelium of the anterior BRB.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannella B., Cross A. H., Raine C. S. Anti-adhesion molecule therapy in experimental autoimmune encephalomyelitis. J Neuroimmunol. 1993 Jul;46(1-2):43–55. doi: 10.1016/0165-5728(93)90232-n. [DOI] [PubMed] [Google Scholar]
- Chang C. W., Roque R. S., Defoe D. M., Caldwell R. B. An improved method for isolation and culture of pigment epithelial cells from rat retina. Curr Eye Res. 1991 Nov;10(11):1081–1086. doi: 10.3109/02713689109020348. [DOI] [PubMed] [Google Scholar]
- Devine L., Lightman S., Greenwood J. Lymphocyte migration across the anterior and posterior blood-retinal barrier in vitro. Cell Immunol. 1996 Mar 15;168(2):267–275. doi: 10.1006/cimm.1996.0075. [DOI] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Pavilack M. A., Todd R. F., 3rd, Mayo-Bond L., Franklin W. A., Strieter R. M., Kunkel S. L., Huber A. R. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992 Feb;66(2):200–211. [PubMed] [Google Scholar]
- Greenwood J., Calder V. L. Lymphocyte migration through cultured endothelial cell monolayers derived from the blood-retinal barrier. Immunology. 1993 Nov;80(3):401–406. [PMC free article] [PubMed] [Google Scholar]
- Greenwood J., Howes R., Lightman S. The blood-retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 1994 Jan;70(1):39–52. [PubMed] [Google Scholar]
- Greenwood J., Wang Y., Calder V. L. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. off. Immunology. 1995 Nov;86(3):408–415. [PMC free article] [PubMed] [Google Scholar]
- Hauzenberger D., Klominek J., Sundqvist K. G. Functional specialization of fibronectin-binding beta 1-integrins in T lymphocyte migration. J Immunol. 1994 Aug 1;153(3):960–971. [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Wykretowicz A. Effect of a new monoclonal antibody, TA-2, that inhibits lymphocyte adherence to cytokine stimulated endothelium in the rat. J Immunol. 1991 Jul 1;147(1):109–116. [PubMed] [Google Scholar]
- Li Y. Y., Cheung H. T. Basement membrane and its components on lymphocyte adhesion, migration, and proliferation. J Immunol. 1992 Nov 15;149(10):3174–3181. [PubMed] [Google Scholar]
- Liversidge J., Sewell H. F., Forrester J. V. Interactions between lymphocytes and cells of the blood-retina barrier: mechanisms of T lymphocyte adhesion to human retinal capillary endothelial cells and retinal pigment epithelial cells in vitro. Immunology. 1990 Nov;71(3):390–396. [PMC free article] [PubMed] [Google Scholar]
- Mesri M., Liversidge J., Forrester J. V. ICAM-1/LFA-1 interactions in T-lymphocyte activation and adhesion to cells of the blood-retina barrier in the rat. Immunology. 1994 Sep;83(1):52–57. [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Look D. C., Roswit W. T., Bragdon M. J., Holtzman M. J. Selective differences in vascular endothelial- vs. airway epithelial-T cell adhesion mechanisms. Am J Physiol. 1994 Oct;267(4 Pt 1):L422–L432. doi: 10.1152/ajplung.1994.267.4.L422. [DOI] [PubMed] [Google Scholar]
- Osusky R., Soriano D., Ye J., Ryan S. J. Cytokine effect on fibronectin release by retinal pigment epithelial cells. Curr Eye Res. 1994 Aug;13(8):569–574. doi: 10.3109/02713689408999890. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., MacPhee I. A., Puklavec M. Isolation of encephalitogenic CD4+ T cell clones in the rat. Cloning methodology and interferon-gamma secretion. J Immunol Methods. 1989 Jul 26;121(2):185–196. doi: 10.1016/0022-1759(89)90159-2. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Uchio E., Kijima M., Tanaka S., Ohno S. Suppression of experimental uveitis with monoclonal antibodies to ICAM-1 and LFA-1. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2626–2631. [PubMed] [Google Scholar]
- Wang Y., Calder V. L., Lightman S. L., Greenwood J. Antigen presentation by rat brain and retinal endothelial cells. J Neuroimmunol. 1995 Sep;61(2):231–239. doi: 10.1016/0165-5728(95)00096-k. [DOI] [PubMed] [Google Scholar]
- Welsh C. T., Rose J. W., Hill K. E., Townsend J. J. Augmentation of adoptively transferred experimental allergic encephalomyelitis by administration of a monoclonal antibody specific for LFA-1 alpha. J Neuroimmunol. 1993 Mar;43(1-2):161–167. doi: 10.1016/0165-5728(93)90087-f. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Zhao Z. S., Calder V. L., McLauchlan M., Lightman S. L. Differential lymphokine expression by rat antigen-specific CD4+ T cell lines with antigen and mitogen. Cell Immunol. 1994 Dec;159(2):220–234. doi: 10.1006/cimm.1994.1309. [DOI] [PubMed] [Google Scholar]