Abstract
An intravenous injection of Rhodococcus aurantiacus to mice causes granulomatous inflammation dependent on endogenous interferon-gamma (IFN-gamma). The present study examined the role of endogenous interleukin-4 (IL-4) on granulomatous inflammation. Endogenous IL-4 in the spleen extracts was not detected during the phase of granuloma formation by enzyme-linked immunosorbent assay (ELISA). However, IL-4 protein level was elevated during the phase of granuloma regression. IL-4 mRNA expression in the livers and spleens was also elevated during the phase of granuloma regression. In addition, IL-4 levels during the phase of granuloma formation were increased by treatment with anti-IFN-gamma monoclonal antibody (mAb), suggesting that endogenous IFN-gamma might inhibit IL-4 production during the phase of granuloma formation. Administration of anti-IL-4 mAb on weeks 3 and 4 after the inoculation inhibited the regression of granulomas and augumented IFN-gamma level at 5 weeks. Endogenous IFN-gamma was produced by CD4+ T cells during the phase of granuloma regression and endogenous IL-4 was produced by both CD4+ and CD8+ T cells. These findings suggest that during the phase of granuloma formation endogenous IL-4 might be inhibited by IFN-gamma, while during the phase of granuloma regression endogenous IL-4 might play a crucial role in the reduction of granulomas and IFN-gamma production.
Full text
PDF

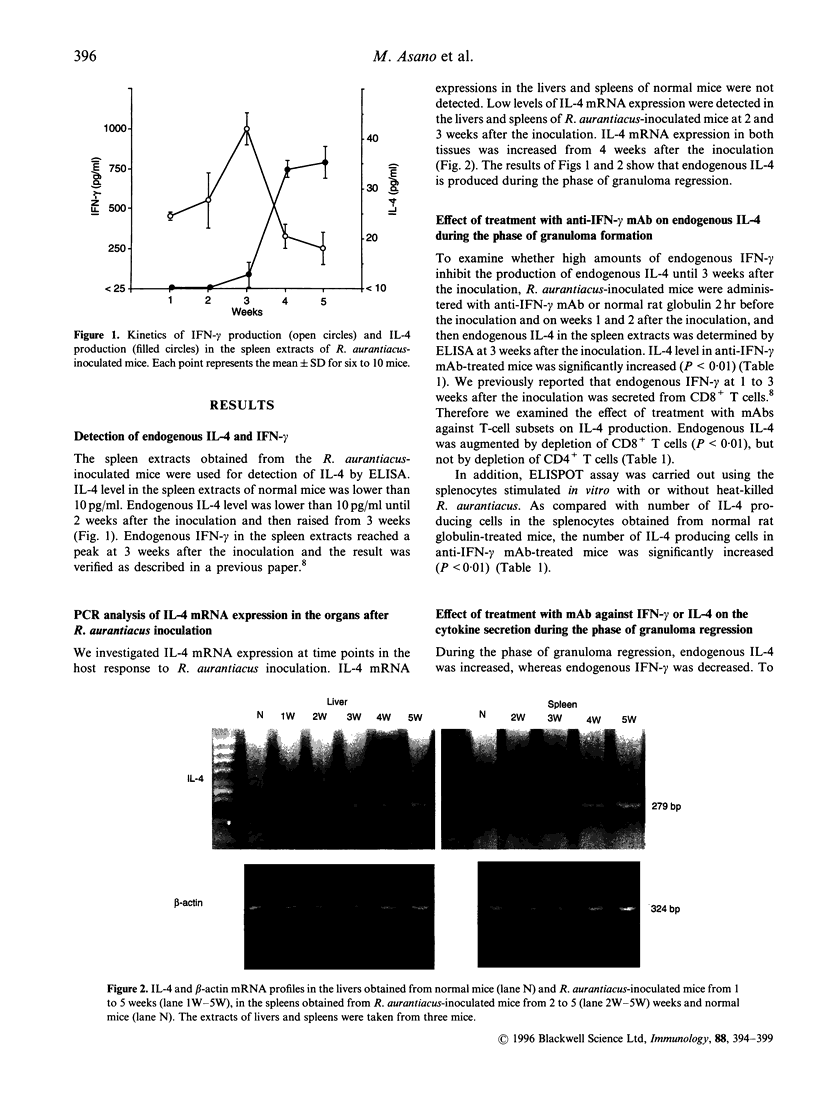
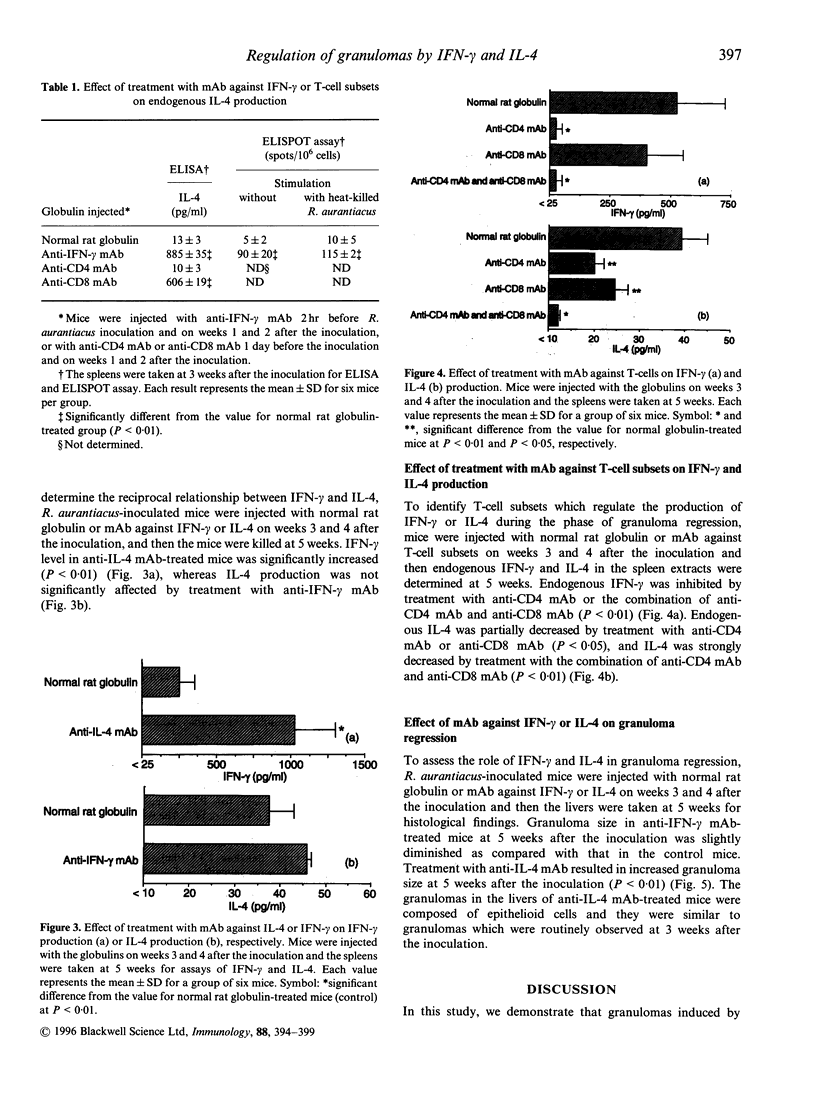
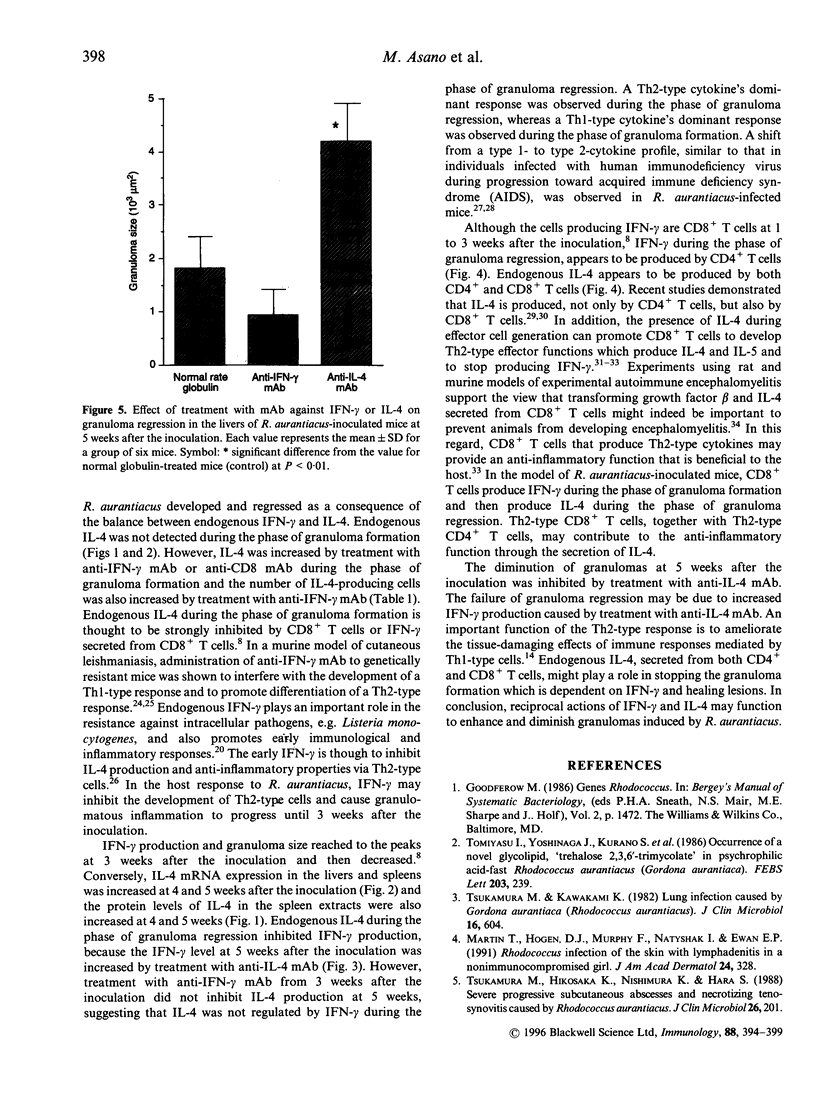

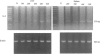
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Minagawa T., Ohmichi M., Hiraga Y. Detection of endogenous cytokines in sera or in lymph nodes obtained from patients with sarcoidosis. Clin Exp Immunol. 1991 Apr;84(1):92–96. doi: 10.1111/j.1365-2249.1991.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M., Nakane A., Kohanawa M., Minagawa T. Sequential involvement of NK cells and CD8+ T cells in granuloma formation of Rhodococcus aurantiacus-infected mice. Microbiol Immunol. 1995;39(7):499–507. doi: 10.1111/j.1348-0421.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- Asano M., Nakane A., Minagawa T. Endogenous gamma interferon is essential in granuloma formation induced by glycolipid-containing mycolic acid in mice. Infect Immun. 1993 Jul;61(7):2872–2878. doi: 10.1128/iai.61.7.2872-2878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994 Dec;15(12):575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Croft M., Carter L., Swain S. L., Dutton R. W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994 Nov 1;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Erard F., Wild M. T., Garcia-Sanz J. A., Le Gros G. Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science. 1993 Jun 18;260(5115):1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- Haak-Frendscho M., Brown J. F., Iizawa Y., Wagner R. D., Czuprynski C. J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992 Jun 15;148(12):3978–3985. [PubMed] [Google Scholar]
- Havell E. A. Purification and further characterization of an anti-murine interferon-gamma monoclonal neutralizing antibody. J Interferon Res. 1986 Oct;6(5):489–497. doi: 10.1089/jir.1986.6.489. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Noble A., Holmes B. J., Diaz-Sanchez D. Immune regulation: a new role for the CD8+ T cell. Immunol Today. 1994 Mar;15(3):107–110. doi: 10.1016/0167-5699(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Maggi E., Giudizi M. G., Biagiotti R., Annunziato F., Manetti R., Piccinni M. P., Parronchi P., Sampognaro S., Giannarini L., Zuccati G. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994 Aug 1;180(2):489–495. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T., Hogan D. J., Murphy F., Natyshak I., Ewan E. P. Rhodococcus infection of the skin with lymphadenitis in a nonimmunocompromised girl. J Am Acad Dermatol. 1991 Feb;24(2 Pt 2):328–332. doi: 10.1016/0190-9622(91)70044-3. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nabors G. S., Farrell J. P. Depletion of interleukin-4 in BALB/c mice with established Leishmania major infections increases the efficacy of antimony therapy and promotes Th1-like responses. Infect Immun. 1994 Dec;62(12):5498–5504. doi: 10.1128/iai.62.12.5498-5504.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Asano M., Kohanawa M., Chen Y., Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990 Jul;58(7):2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Numata A., Chen Y., Minagawa T. Endogenous gamma interferon-independent host resistance against Listeria monocytogenes infection in CD4+ T cell- and asialo GM1+ cell-depleted mice. Infect Immun. 1991 Oct;59(10):3439–3445. doi: 10.1128/iai.59.10.3439-3445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994 Jan 28;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Prinz G., Bán E., Fekete S., Szabó Z. Meningitis caused by Gordona aurantiaca (Rhodococcus aurantiacus). J Clin Microbiol. 1985 Sep;22(3):472–474. doi: 10.1128/jcm.22.3.472-474.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Boulay J. L., Finkelman F., Barbier S., Ben-Sasson S. Z., Le Gros G., Paul W. E. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992 Mar 15;148(6):1652–1656. [PubMed] [Google Scholar]
- Tsukamura M., Hikosaka K., Nishimura K., Hara S. Severe progressive subcutaneous abscesses and necrotizing tenosynovitis caused by Rhodococcus aurantiacus. J Clin Microbiol. 1988 Feb;26(2):201–205. doi: 10.1128/jcm.26.2.201-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M., Kawakami K. Lung infection caused by Gordona aurantiaca (Rhodococcus aurantiacus). J Clin Microbiol. 1982 Oct;16(4):604–607. doi: 10.1128/jcm.16.4.604-607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Friedman A., Miller A., Khoury S. J., al-Sabbagh A., Santos L., Sayegh M., Nussenblatt R. B., Trentham D. E., Hafler D. A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]