Abstract
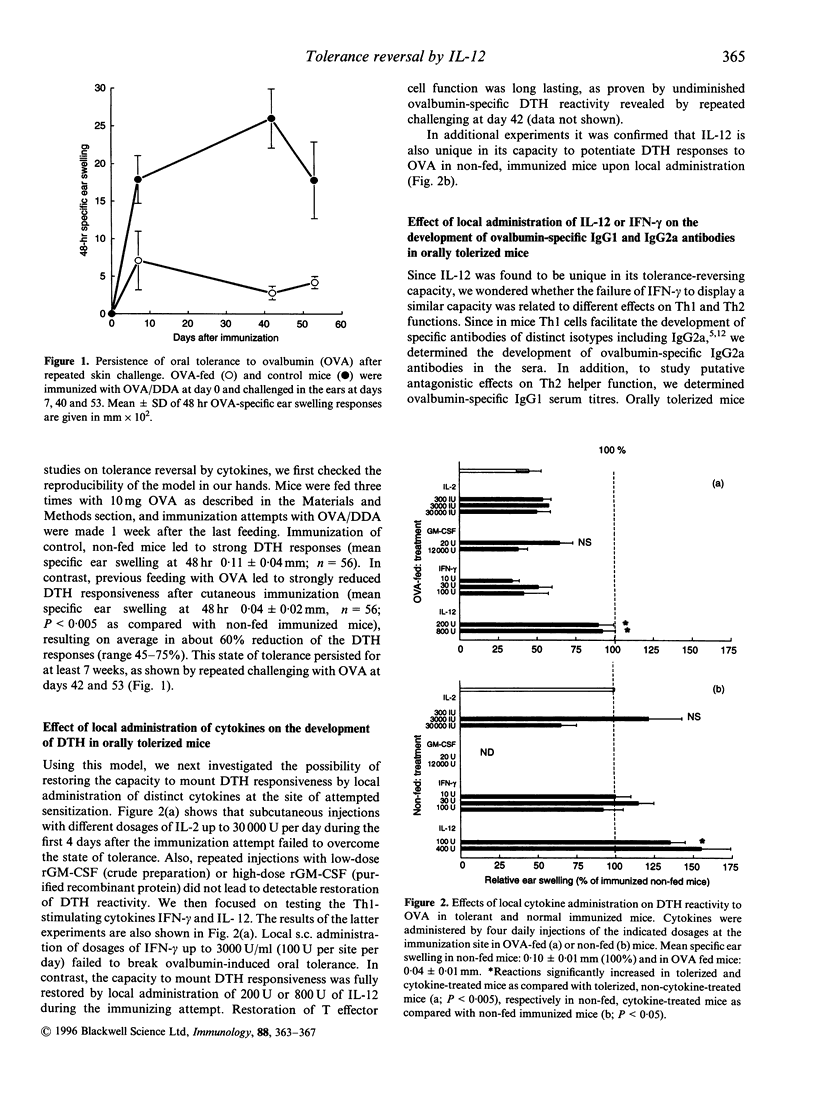
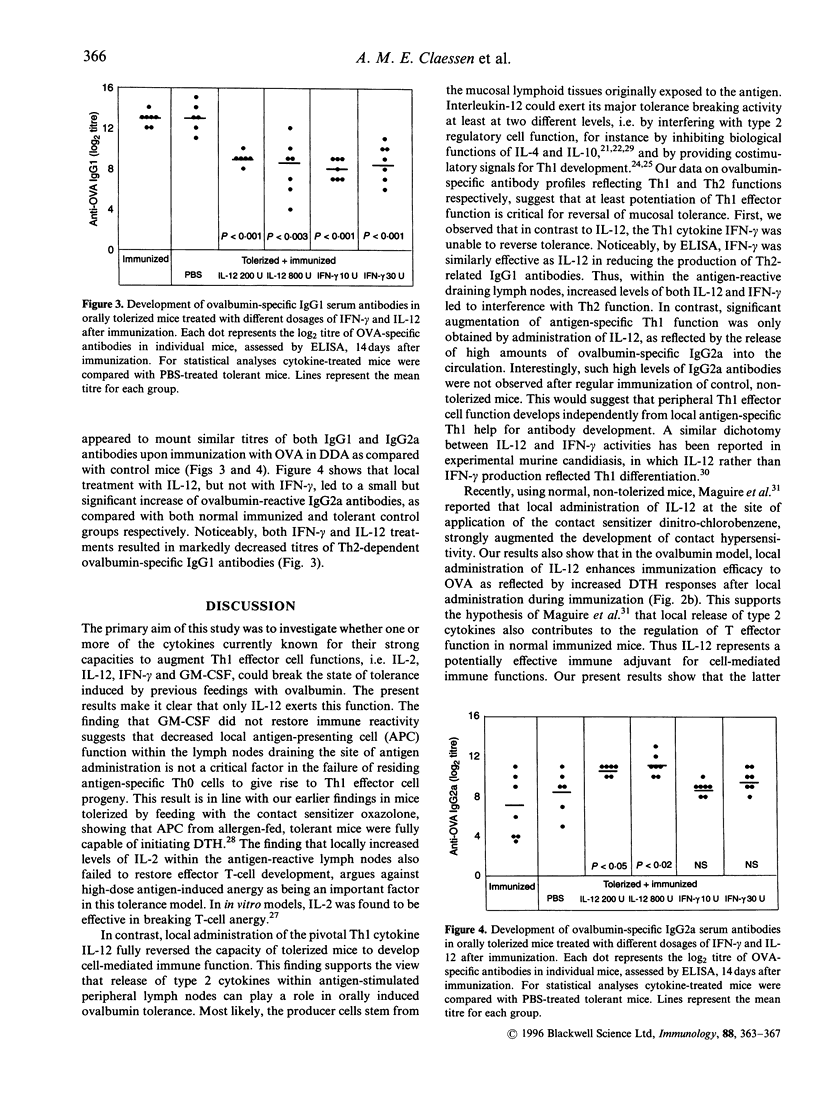
Oral feeding of proteins causes peripheral T-cell tolerance, as revealed by reduced delayed-type hypersensitivity (DTH) reactivity after immunization. This type of tolerance can be due both to passive T-cell anergy and active immunosuppression. Using ovalbumin-fed mice we studied whether putatively immunostimulatory cytokines could break this state of mucosal tolerance. Cytokines were administered locally at the site of attempted sensitization. It was found that neither interleukin-2 (IL-2), interferon-gamma (IFN-gamma) nor granulocyte-macrophage colony-stimulating factor (GM-CSF) could restore the response to immunization. In contrast, local administration of IL-12 at the site of attempted immunization resulted in full recovery of DTH reactivity. The dichotomy between the two Th1 stimulatory cytokines IFN-gamma and IL-12 was also reflected by different effects on ovalbumin-specific antibody isotypes. Although both IFN-gamma and IL-12 downregulated serum IgG1-levels in tolerant mice, suggesting decreased ovalbumin-specific Th2 function, only local administration of IL-12 led to increased serum IgG2a levels. These results support the view that potentiation of Th1 effector function is critical for reversal of mucosal tolerance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill F. Cytokines--soluble factors in immune responses. Curr Opin Immunol. 1988 Dec;1(2):241–249. doi: 10.1016/0952-7915(88)90008-8. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Abbas A. K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993 Feb 1;177(2):457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Lancki D. W., Stack R., Fitch F. W. "Anergy" of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994 Feb 1;179(2):481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P., Steel M., Worthey E. A., Satoskar A., Alexander J., Bluethmann H., Liew F. Y., Mowat A. M. T helper 2 cells are subject to high dose oral tolerance and are not essential for its induction. J Immunol. 1995 Jun 1;154(11):5649–5655. [PubMed] [Google Scholar]
- Gregerson D. S., Obritsch W. F., Donoso L. A. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993 Nov 15;151(10):5751–5761. [PubMed] [Google Scholar]
- Ishii N., Moriguchi N., Sugita Y., Nakajima H., Tanaka S., Aoki I. Analysis of responsive cells in tolerance by the oral administration of ovalbumin. Immunol Invest. 1993 Aug-Oct;22(6-7):451–462. doi: 10.3109/08820139309063423. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Noble A., Holmes B. J., Diaz-Sanchez D. Immune regulation: a new role for the CD8+ T cell. Immunol Today. 1994 Mar;15(3):107–110. doi: 10.1016/0167-5699(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Kharkevitch D. D., Seito D., Balch G. C., Maeda T., Balch C. M., Itoh K. Characterization of autologous tumor-specific T-helper 2 cells in tumor-infiltrating lymphocytes from a patient with metastatic melanoma. Int J Cancer. 1994 Aug 1;58(3):317–323. doi: 10.1002/ijc.2910580302. [DOI] [PubMed] [Google Scholar]
- Kubin M., Kamoun M., Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994 Jul 1;180(1):211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens J., Scheper R. J. Synergistic effects of locally administered cytostatic drugs and a surfactant on the development of delayed-type hypersensitivity to keyhole limpet haemocyanin in mice. Clin Exp Immunol. 1989 Nov;78(2):256–262. [PMC free article] [PubMed] [Google Scholar]
- Lombardi G., Sidhu S., Batchelor R., Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994 Jun 10;264(5165):1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- Lüscher U., Filgueira L., Juretic A., Zuber M., Lüscher N. J., Heberer M., Spagnoli G. C. The pattern of cytokine gene expression in freshly excised human metastatic melanoma suggests a state of reversible anergy of tumor-infiltrating lymphocytes. Int J Cancer. 1994 May 15;57(4):612–619. doi: 10.1002/ijc.2910570428. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Zimmer G. J., Fogelman I., Wolf S. F., Abbas A. K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994 Mar 1;152(5):2172–2179. [PubMed] [Google Scholar]
- Migita K., Ochi A. The fate of anergic T cells in vivo. J Immunol. 1993 Feb 1;150(3):763–770. [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Mosmann T. R. Cytokines: is there biological meaning? Curr Opin Immunol. 1991 Jun;3(3):311–314. doi: 10.1016/0952-7915(91)90029-z. [DOI] [PubMed] [Google Scholar]
- Mowat A. M. The role of antigen recognition and suppressor cells in mice with oral tolerance to ovalbumin. Immunology. 1985 Oct;56(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Sep;23(9):2223–2229. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the development of type 2 T-helper cells in allergy. Curr Opin Immunol. 1994 Dec;6(6):838–846. doi: 10.1016/0952-7915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Tonnetti L., Spaccapelo R., Cenci E., Wolf S., Puccetti P., Bistoni F. Interleukin-12 but not interferon-gamma production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur J Immunol. 1994 Apr;24(4):909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- Schmitt E., Hoehn P., Germann T., Rüde E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994 Feb;24(2):343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- Schoof D. D., Terashima Y., Peoples G. E., Goedegebuure P. S., Andrews J. V., Richie J. P., Eberlein T. J. CD4+ T cell clones isolated from human renal cell carcinoma possess the functional characteristics of Th2 helper cells. Cell Immunol. 1993 Aug;150(1):114–123. doi: 10.1006/cimm.1993.1183. [DOI] [PubMed] [Google Scholar]
- Suzuki G., Nomura M., Uzawa A., Akashi M., Nakata Y. Impaired CD28-mediated co-stimulation in anergic T cells. Int Immunol. 1995 Jan;7(1):37–43. doi: 10.1093/intimm/7.1.37. [DOI] [PubMed] [Google Scholar]
- Tahara H., Zitvogel L., Storkus W. J., Zeh H. J., 3rd, McKinney T. G., Schreiber R. D., Gubler U., Robbins P. D., Lotze M. T. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995 Jun 15;154(12):6466–6474. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994 Dec 15;84(12):4008–4027. [PubMed] [Google Scholar]
- Van Wilsem E. J., Van Hoogstraten I. M., Brevé J., Scheper R. J., Kraal G. Dendritic cells of the oral mucosa and the induction of oral tolerance. A local affair. Immunology. 1994 Sep;83(1):128–132. [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Friedman A., Miller A., Khoury S. J., al-Sabbagh A., Santos L., Sayegh M., Nussenblatt R. B., Trentham D. E., Hafler D. A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Sieburth D., Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells. 1994 Mar;12(2):154–168. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- Xu-Amano J., Jackson R. J., Fujihashi K., Kiyono H., Staats H. F., McGhee J. R. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994 Aug;12(10):903–911. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Kato T., Igarashi O., Inoue T., Nariuchi H. Second signal activity of IL-12 on the proliferation and IL-2R expression of T helper cell-1 clone. J Immunol. 1994 May 15;152(10):4919–4928. [PubMed] [Google Scholar]
- van Hoogstraten I. M., von Blomberg B. M., Boden D., Kraal G., Scheper R. J. Non-sensitizing epicutaneous skin tests prevent subsequent induction of immune tolerance. J Invest Dermatol. 1994 Jan;102(1):80–83. doi: 10.1111/1523-1747.ep12371736. [DOI] [PubMed] [Google Scholar]


