Abstract
The MtArg8 reversion assay, which measures point mutation in mtDNA, indicates that in budding yeast Saccharomyces cerevisiae, DNA polymerase ζ and Rev1 proteins participate in the mitochondrial DNA mutagenesis. Supporting this evidence, both polymerase ζ and Rev1p were found to be localized in the mitochondria. This is the first report demonstrating that the DNA polymerase ζ and Rev1 proteins function in the mitochondria.
A number of human diseases, including cancer, have been attributed to pathogenic mutations of mitochondrial DNA (mtDNA) (Naviaux 2000, 2004; Modica-Napolitano and Singh 2002, 2004; Kang and Hamasaki 2005). Although mitochondria contain their own DNA encoding a handful of proteins, most mitochondrial proteins are synthesized in the cytoplasm and imported into the organelle (Jensen and Dunn 2002). The import system is complex and includes, for most proteins, a targeting sequence at the N terminus (Haucke and Schatz 1997). mtDNA is continuously subjected to damage by reactive oxygen species (ROS), which are produced in the mitochondria as a byproduct of oxidative phosphorylation (OXPHOS) (Chi and Kolodner 1994; Croteau and Bohr 1997). Consistently, the accumulation of mutations in mtDNA is ∼10-fold greater than that in nuclear DNA, due to the proximity of mtDNA to ROS and the lack of protective histones (Yakes and Houten 1997; Singh et al. 2001). The limited mtDNA repair also contributes to the accumulation of mtDNA mutation. In most organisms mitochondria depend on nuclear-encoded proteins to repair their DNA. In this regard, our previous studies suggest that uracil–DNA glycosylase, encoded by the UNG1 gene in budding yeast, is capable of repairing uracil in mtDNA formed due to deamination of cytosine (Chatterjee and Singh 2001). In another study, we showed that 8-oxo-G encoded by the OGG-1 gene also localized to mitochondria and repaired mtDNA (Singh et al. 2001). Research efforts from other laboratories have also contributed to better understanding of the mechanisms underlying DNA repair in mitochondria (Bogenhagen 1999; Doudican et al. 2005; Rasmussen and Rasmussen 2005; Stuart et al. 2005).
Translesion DNA synthesis (TLS) is an important damage bypass system known to operate in the nucleus. TLS enables cells to bypass replication, blocking oxidative and other lesions in the nuclear DNA (Gibbs et al. 1998; Lawrence 2004). TLS is mutagenic because it often incorporates incorrect nucleotides and is therefore described as an error-prone DNA translesion synthesis pathway (Nair et al. 2005; Prakash et al. 2005). Three proteins, Rev1, Rev3, and Rev7, constitute the major components of the error-prone TLS (Nelson et al. 1996). Rev1 belongs to the UmuC family of proteins and possesses a deoxycytidyl (dCMP) transferase activity in a template-dependent reaction, which can efficiently insert a dCMP opposite a template apurinic/apyrimidinic (AP) site, whereas the Rev3 and Rev7 proteins constitute DNA polymerase ζ (Pol ζ) (Kozmin et al. 2003; Lawrence 2004). The human homologs of these proteins have been identified (Morelli et al. 1998; Lin et al. 1999; Gibbs et al. 2000; Murakumo et al. 2000, 2001). These proteins are responsible for the majority of spontaneous and damage-induced DNA mutagenesis in the nucleus.
In this article, we provide evidence that yeast TLS proteins polymerase ζ and Rev1p localize to mitochondria. Furthermore, we demonstrate that inactivation of REV3 and REV7 encoding polymerase ζ, as well as of REV1 genes, leads to suppression of mutation in mtDNA.
Yeast polymerase ζ and Rev1p localize in the mitochondria:
Using the PSORT II (http://psort.nibb.ac.jp) software designed to identify mitochondrial targeting signal (MTS) in a protein, we analyzed the amino terminus of Rev1, Rev3, and Rev7 proteins. Our analysis suggested that these proteins contain putative MTS at their N termini. On the basis of these predictions, DNA encoding N-terminal amino acids was amplified by PCR and fused individually in frame with the pGFP-C-Fus plasmid DNA encoding green fluorescent protein (GFP) (Niedenthal et al. 1996). Mitotracker dye was used to locate the mitochondrial compartments (Figure 1A). The merged images clearly show that the fusion proteins yREV1-GFP, yREV3-GFP, and yREV7-GFP (Figure 1) localize to the mitochondria, whereas the GFP-encoding plasmid is distributed evenly in the cytoplasm. This study reveals that the N-terminal amino acids 1–148, 1–115, and 1–106, from the yeast Rev1p, Rev3p, and Rev7p, respectively, can direct GFP protein into the mitochondria. To further substantiate our finding, we carried out Western blot analysis of mitochondrial extracts prepared from strains expressing the fusion proteins. Figure 1B shows a single band of ∼58 kDa GFP protein. As a positive control, we stripped membrane and reprobed it with an antibody against Mas2p (53.2 kDa), an authentic mitochondrial protein. Together, these studies demonstrate that polymerase ζ and Rev1p are indeed mitochondrial proteins.
Figure 1.
Subcellular localization of yeast REV gene products. (A) The yeast strain YF250 (MATα ura3-52 hisΔ200 leu2Δ1 trp1Δ63) cells expressing fusion protein yREV1-GFP (1–148 bp), yREV3-GFP (1–115 bp), or yREV7-GFP (1–106 bp) were grown in selection media lacking uracil and methionine. Cells were then examined by routine fluorescent microscopy and Mitotracker dye was used to locate the subcellular mitochondria. Merged images show that these fusion proteins localize to the mitochondria, revealing that yeast REV1, REV3, and REV7 gene products are localized in the mitochondria. The targeting sequence-containing plasmids encoding these fusion proteins were constructed using the vector pGFP-C-Fus and the primers listed in Table 1. (B) Mitochondria were isolated from the yeast strain YF250 transformed with the plasmid constructs pGFP-REV1, pGFP-REV3, and pGFP-REV7, and Western blot analysis of the extracted proteins was performed. The top row shows the bands probed with GFP antiserum. The bottom row shows the bands recognized by Mas2 antibody. Lanes 1–3 were loaded with the different fusion proteins as indicated.
Inactivation of genes encoding polymerase ζ and Rev1p decreases the frequency of mtDNA mutation:
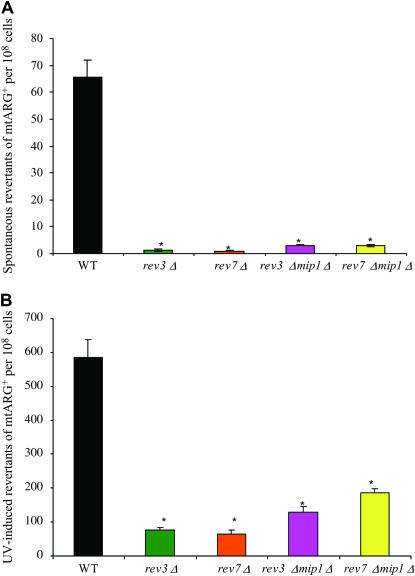
The above studies clearly demonstrate that yeast Pol ζ and Rev1p localize in the mitochondria. We therefore tested whether these proteins were involved in spontaneous or induced mutagenesis of the mitochondrial genome. Yeast REV1, REV3, or REV7 genes were inactivated and the frequency of mtDNA mutation was measured by mtarg8 reversion assay as described by Strand and Copeland (2002). This assay is based on the fact that a yeast strain containing the mitochondrial ARG8 gene is auxotrophic for arginine because the mtarg8 gene contains two point mutations plus a +1 frameshift mutation, the reversion of which (−1 frameshift mutation) is the basis of the assay. Reversion gives rise to the arg+ phenotype, which can be selected using media lacking arginine. Our study revealed that the frequency of spontaneous mtDNA mutation in rev3 and rev7 single mutants was significantly reduced when compared to that in the wild type (Figure 2A). Consistently, UV-induced frequency of mtDNA mutation was also reduced in rev3 and rev7 single mutants (Figure 2B). The spontaneous and UV-induced frequency of mtDNA mutation in the rev1 mutant was also reduced when compared to that in the wild type (Figure 3, A and B). We conclude that the yeast Pol ζ and Rev1p operate in the mitochondria and contribute to mitochondrial genome mutagenesis in a way analogous to its function in nucleus.
Figure 2.
Frequency of mtDNA mutations in TF236 [MATα ino1∷HIS3 arg8∷HISG pet9 (op1) ura3-52 lys2 cox3∷mtarg8] and its mutant derivatives. The strain TF236 was kindly provided by W. C. Copeland (National Institute of Environmental Health Sciences), and deletions of REV, REV3, REV7, and MIP1 were introduced as described (Foury 1989; Rasmussen et al. 2003), using suitable gene knockout constructs provided by C. W. Lawrence (University of Rochester, Rochester, NY) and F. Foury (Université de Louvain, Belgium). The mutant strains were verified by PCR. The cox3∷mtarg8 construct in the strain TF236 was generated by T. D. Fox's group, who recoded the nuclear ARG8 gene using the mitochondrial genetic code and inserted this gene into the COX3 gene in the mitochondrial genome (Steele et al. 1996). Frequency of spontaneous mtDNA (A) was determined by using the mtarg8 reversion assay as described (see text and Strand and Copeland 2002 for details). When the frequency of UV-induced mtDNA mutation was determined (B), the same yeast strains were grown for 2 days to stationary phase at 30° in a rotary shaker in YPD medium. They were then washed and diluted appropriately, followed by plating them in duplicate to YPD and SD/Arg− plates. The plated cells in YPD were incubated at 30° for 3 days to obtain viable counts, and those in medium lacking arginine were irradiated with UVC at a 35 J/m2 dose using UV Stratalinker (Stratagene, La Jolla, CA) and then incubated in the dark at 30° for 3 weeks to obtain arg+ revertant counts. In both A and B, values presented are the average ± SEM of 20 independent cultures beginning with single colonies and are compared statistically, using Student's t-test assuming unequal variances. *P < 0.01.
Figure 3.
Frequency of mtDNA mutation in TF236 and its mutant derivatives. Materials and methods used are the same as those described in Figure 2. In both spontaneous (A) and UV-induced (B) revertants, values presented are the average ± SEM of 20 independent cultures beginning with single colonies and are compared statistically using Student's t-test assuming unequal variances. *P < 0.01.
Polymerase ζ and γ belong to same epistatic pathways:
To date, DNA polymerase γ (Pol γ), encoded by the MIP1 gene in yeast, is the only polymerase described in the mitochondria (Foury 1989). Disruption of the MIP1 gene demonstrates that the enzyme is required for mtDNA replication (Schultz et al. 1998; Chan et al. 2005). Apart from its role in mtDNA replication, Pol γ also plays a part in mtDNA repair (Bogenhagen 1999; Chan et al. 2005). We used a mitochondrial mutation assay developed by Strand and Copeland (2002) and Strand et al. (2003) to investigate whether Pol ζ and Pol γ belong to same or different genetic pathways. This mitochondrial mutation assay uses a strain (TF236) in which the PET9 gene is inactivated so that it does not permit loss of mtDNA. We found no further decrease in the frequency of mtDNA mutation in mip1rev3 or mip1rev7 double mutants compared to that in the rev3 or rev7 single mutant (Figure 2, A and B), suggesting that Pol γ and Pol ζ belong to the same epistatic group. Interestingly, the drop in the frequency of spontaneous mtDNA mutation in the mip1 rev1 double mutant was more compared to that in the rev1 single mutant (Figure 3A). A similar drop in the frequency of mtDNA mutation was obtained in response to UV (Figure 3B). These results suggest that Rev1p belongs to a different epistatic group when compared with Mip1p. These results provide evidence for the existence of complex interactions among polymerase ζ, Rev1p, and Mip1 proteins and underscore the complexity of underlying mechanisms in maintenance of mtDNA stability.
Human REV1p, REV3p, and REV7p do not localize to the mitochondria:
The human homologs of the yeast proteins involved in error-prone TLS have been identified (Gibbs et al. 1998; Lin et al. 1999; Murakumo 2002). The human cells expressing hREV1 or hREV3 antisense mRNA show less mutagenic properties after UV irradiation, suggesting that hREV1 and hREV3 are involved in UV-induced TLS mutagenesis (Gibbs et al. 1998; Kozmin et al. 2003). Recombinant hREV1 protein shows terminal deoxycytidyl transfererse activity (Lin et al. 1999), as does yeast Rev1 protein. Interaction between hREV3 and hREV7 indicates the existence of DNA polymerase ζ complex in human cells (Murakumo et al. 2001). To identify a role for human REV proteins in the mtDNA mutagenesis, we examined the mitochondrial localization of the human REV gene products by fluorescent confocal microscopy. On the basis of the fact that the three yeast REV gene products localize to the mitochondria, we determined whether or not this is true for the human homologs. We cloned the DNA sequence encoding the N terminus containing putative MTS from hREV1, hREV3, or hREV7 in frame with GFP in the pEGFP-N2 plasmid. The fusion construct was transiently transfected in the MDA-MB-435 human cell line. In contrast to yeast, the human REV1 fusion protein was predominantly localized to the nucleus, whereas the human REV3 and REV7 proteins were found in the cytoplasm. No mitochondrial distribution was detected for any of the three human REV proteins (Figure 4). These studies suggest that the N termini of these human REV proteins do not contain the properties of the mitochondrial targeting signal.
Figure 4.
Subcellular localization of the human REV gene products. The cultured human breast carcinoma cell line MDA-MB-435 was transiently transfected with each of the constructs, including hREV1-pEGFP, hREV3-pEGFP, and hREV7-pEGFP, separately and subcellular localization of the expressed fusion proteins was examined using fluorescent confocal microscopy. Mitotracker dye was used to locate the subcellular mitochondria. Merged images show that these fusion proteins localize to different subcellular compartments. The targeting sequence-containing plasmids encoding these fusion proteins were constructed using the vector pEGFP-N2 and the primers listed in Table 1. The plasmids containing the cDNA sequences encoding the human REV1, REV3, or REV7 were kindly provided by C. W. Lawrence (University of Rochester, Rochester, NY).
TABLE 1.
Primers used to make fusion proteins in this study
| Name | Sequence (5′–3′) |
|---|---|
| Yeast gene | |
| yREV1 forward | CGGACTAGTATGGGTGAACATGGTGGTCTTG |
| yREV1 backward | CCCGGAATTCTTGATCTTGCAGGGTTTGTCCG |
| yREV3 forward | CAGCGGATCCATGTCGAGGGAGTCGAACGACACAATA |
| yREV3 backward | CCCGGAATTCAATTTTTACTTCCAGCG |
| yREV7 forward | CCGCGGATCCATGAATAGATGGGTAGAG |
| yREV7 backward | CCCGGAATTCCTGATCGTCTTTATCCACAT |
| Human gene | |
| hREV1 forward | GCCGGCTCGAGCATGAGGCGAGGTGGATGGAGGAAG |
| hREV1 backward | CCGGCGAATTCAATTTTGGCATTGGGAAGATTTGTGGC |
| hREV3 forward | GCCGGCTCGAGCATGTTTTCAGTAAGGATAGTGACTGC |
| hREV3 backward | CCGGCGAATTCATGCTGAGCAGTGGAAGATGG |
| hREV7 forward | GCCGGCTCGAGCATGACCACGCTCACACGAC |
| hREV7 backward | CCGGCGAATTCAAAGACGAATTTCTCCACTGG |
Acknowledgments
We thank W. C. Copeland (National Institute of Environmental Health Sciences) for yeast strain TF236, Ed Hurley for help with confocal microscopy, and Ellen S. Sanders-Noonan for editing this manuscript. This study was supported by grants ES-097714 and CA113655 from the National Institutes of Health and by the Charlotte Geyer Foundation.
References
- Bogenhagen, D. F., 1999. Repair of mtDNA in vertebrates. Am. J. Hum. Genet. 64: 1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. S., M. J. Longley and W. C. Copeland, 2005. The common A467T mutation in the human mitochondrial DNA polymerase (POLG) compromises catalytic efficiency and interaction with the accessory subunit. J. Biol. Chem. 280: 31341–31346. [DOI] [PubMed] [Google Scholar]
- Chatterjee, A., and K. K Singh, 2001. Uracil-DNA glycosylase-deficient yeast exhibits a mitochondrial mutator phenotype. Nucleic Acids Res. 29: 4935–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, N.W., and R. D. Kolodner, 1994. Purification and characterization of MSH1, yeast mitochondrial protein that binds to DNA mismatches. J. Biol. Chem. 269: 29984–29992. [PubMed] [Google Scholar]
- Croteau, D. L., and V. A. Bohr, 1997. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J. Biol. Chem. 272: 25409–25412. [DOI] [PubMed] [Google Scholar]
- Doudican, N. A., B. Song, G. S. Shadel and P. W. Doetsch, 2005. Oxidative DNA damage causes mitochondrial genomic instability in Saccharomyces cerevisiae. Mol. Cell. Biol. 25(12): 5196–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury, F., 1989. Cloning and sequencing of the nuclear gene MIP1 encoding the catalytic subunit of the yeast mitochondrial DNA polymerase. J. Biol. Chem. 264: 20552–20560. [PubMed] [Google Scholar]
- Gibbs, P. E. M., W. G. McGregor, V. M. Maher, P. Nilsson and C. W. Lawrence, 1998. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase ζ. Proc. Natl. Acad. Sci. USA 95: 6876–6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, P. E. M., X.-D. Wang, Z. Li, T. P. McManus, W. Glenn McGregor et al., 2000. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc. Natl. Acad. Sci. USA 97: 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke, V., and G. Schatz, 1997. Import of proteins into mitochondria and chloroplasts. Trends Cell Biol 7: 103–106. [DOI] [PubMed] [Google Scholar]
- Jensen, R. E., and C. D. Dunn, 2002. Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim. Biophys. Acta 1592: 25–34. [DOI] [PubMed] [Google Scholar]
- Kang, D., and N. Hamasaki, 2005. Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer. Curr. Med. Chem. 12: 429–441. [DOI] [PubMed] [Google Scholar]
- Kozmin, S. G., Y. I. Pavlov, T. A. Kunkel and E. Sage, 2003. Roles of Saccharomyces cerevisiae DNA polymerases Polη and Polζ in response to irradiation by simulated sunlight. Nucleic Acids Res. 31: 4541–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, C. W., 2004. Cellular functions of DNA polymerase ζ and Rev1 protein. Adv. Protein Chem. 69: 167–203. [DOI] [PubMed] [Google Scholar]
- Lin, W., H. Xin, Y. Zhang, X. Wu, F. Yuan et al., 1999. The human REV1 gene codes for a DNA template-dependent dCMP transferase. Nucleic Acids Res. 27: 4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano, J. S., and K. K. Singh, 2002. Mitochondria as targets for detection and treatment of cancer. Exp. Rev. Mol. Med. 2002: 1–19. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano, J. S., and K. K. Singh, 2004. Mitochondrial dysfunction in cancer. Mitochondrion 4: 755–762. [DOI] [PubMed] [Google Scholar]
- Morelli, C., A. J. Mungall, M. Negrini, G. Barbanti-Brodano and C. M. Croce, 1998. Alternative splicing, genomic structure, and fine chromosome localization of REV3L. Cytogenet. Cell Genet. 83: 18–20. [DOI] [PubMed] [Google Scholar]
- Murakumo, Y., 2002. The property of DNA polymerase ζ: REV7 is a putative protein involved in translesion DNA synthesis and cell cycle control. Mutat. Res. 510: 37–44. [DOI] [PubMed] [Google Scholar]
- Murakumo, Y., T. Roth, H. Ishii, D. Rasio, S. I. Numata et al., 2000. A human REV7 homolog that interacts with the Polymerase ζ catalytic subunit hREV3 and the spindle assembly checkpoint protein hMAD2. J. Biol. Chem. 275: 4391–4397. [DOI] [PubMed] [Google Scholar]
- Murakumo, Y., Y. Ogura, H. Ishii, S. I. Numata, M. Ichihara et al., 2001. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J. Biol. Chem. 276: 35644–35651. [DOI] [PubMed] [Google Scholar]
- Nair, D. T., R. E. Johnson, L. Prakash, S. Prakash and A. K. Aggarwal, 2005. Rev1 employs a novel mechanism of DNA synthesis using a protein template. Science 309: 2219–2222. [DOI] [PubMed] [Google Scholar]
- Naviaux, R. K., 2000. Mitochondrial DNA disorders. Eur. J. Pediatr. 159: S219–S226. [DOI] [PubMed] [Google Scholar]
- Naviaux, R. K., 2004. Developing a systematic approach to the diagnosis and classification of mitochondrial disease. Mitochondrion 4: 351–361. [DOI] [PubMed] [Google Scholar]
- Nelson, J. R., C. W. Lawrence and D. C. Hinkle, 1996. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382: 729–731. [DOI] [PubMed] [Google Scholar]
- Niedenthal, R. K., L. Riles, M. Johnston and J. H. Hegemann, 1996. Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast 12: 773–786. [DOI] [PubMed] [Google Scholar]
- Prakash, S., R. E. Johnson and L. Prakash, 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74: 317–353. [DOI] [PubMed] [Google Scholar]
- Rasmussen A. K., and L. J. Rasmussen, 2005. Targeting of O(6)-MeG DNA methyltransferase (MGMT) to mitochondria protects against alkylation induced cell death. Mitochondrion 5: 411–417. [DOI] [PubMed] [Google Scholar]
- Rasmussen, A. K., A. Chatterjee, L. J. Rasmussen and K. K. Singh, 2003. Mitochondria-mediated nuclear mutator phenotype in Saccharomyces cerevisiae. Nucleic Acids Res. 31: 3909–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, R. A., S. J. Swoap, L. D. McDaniel, B. Q. Zhang, E. C. Koon et al., 1998. Differential expression of mitochondrial DNA replication factors in mammalian tissues. J. Biol. Chem. 273: 3447–3451. [DOI] [PubMed] [Google Scholar]
- Singh, K. K., B. Sigala, H. A. Sikder, G. Kim and C. Schwimmer, 2001. Inactivation of Saccharomyces cerevisiae OGG1 gene leads to increased frequency of mitochondrial deletions. Nucleic Acids Res. 29: 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, D. F., C. A. Butler and T. D. Fox, 1996. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc. Natl. Acad. Sci. USA 93: 5253–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, M. K., and W. C. Copeland, 2002. Measuring mtDNA mutation rates in Saccharomyces cerevisiae using the mtarg8 assay. Methods Mol. Biol. 197: 151–157. [DOI] [PubMed] [Google Scholar]
- Strand, M. K., G. R. Stuart, M. J. Longley, M. A. Graziewicz, O. C. Dominick et al., 2003. POS5 gene of Saccharomyces cerevisiae encodes a mitochondrial NADH kinase required for stability of mitochondrial DNA. Eukaryot. Cell 2: 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, J. A., S. Mayard, K. Hashiguchi, N. C. Souza-Pinto and V. A. Bohr, 2005. Localization of mitochondrial DNA base excision repair to an inner membrane-associated particulate fraction. Nucleic Acids Res. 33: 3722–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes, F. M., and B. V. Houten, 1997. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 94: 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]