Abstract
The transcription factor Adr1 directly activates the expression of genes encoding enzymes in numerous pathways that are upregulated after the exhaustion of glucose in the yeast Saccharomyces cerevisiae. ADH2, encoding the alcohol dehydrogenase isozyme required for ethanol oxidation, is a highly glucose-repressed, Adr1-dependent gene. Using a genetic screen we isolated >100 mutants in 12 complementation groups that exhibit ADR1-dependent constitutive ADH2 expression on glucose. Temperature-sensitive alleles are present among the new constitutive mutants, indicating that essential genes play a role in ADH2 repression. Among the genes we cloned is MOT1, encoding a repressor that inhibits TBP binding to the promoter, thus linking glucose repression with TBP access to chromatin. Two genes encoding proteins involved in vacuolar function, FAB1 and VPS35, and CDC10, encoding a nonessential septin, were also uncovered in the search, suggesting that vacuolar function and the cytoskeleton have previously unknown roles in regulating gene expression. Constitutive activation of ADH2 expression by Adr1 is SNF1-dependent in a strain with a defective MOT1 gene, whereas deletion of SNF1 did not affect constitutive ADH2 expression in the mutants affecting vacuolar or septin function. Thus, the mutant search revealed previously unknown Snf1-dependent and -independent pathways of ADH2 expression.
THE yeast Saccharomyces cerevisiae has a sophisticated system for transducing information regarding nutrient availability to the nucleus (Gancedo 1998; Carlson 1999). Glucose, the preferred source of energy, is oxidized to produce carbon dioxide and ethanol during fermentative growth. During this phase of growth the transcription of genes involved in many nonfermentative pathways is repressed, as are genes required for the utilization of less-preferred fermentable substrates, such as galactose, sucrose, and maltose. When glucose is exhausted, these genes are activated to allow the cell to use alternative carbon sources for growth and energy production (Schuller 2003), an adaptive change in metabolism that occurs during the diauxic transition. This change in metabolism is accompanied by a massive reprogramming of gene expression (DeRisi et al. 1997).
The diauxic transition is regulated by the Snf1 protein kinase, the yeast homolog of the mammalian AMP-activated protein kinase (AMPK) (Hardie et al. 1998). Snf1 is part of a kinase complex whose activity is stimulated by low glucose concentration. The activity of the Snf1 kinase complex is regulated by Glc7.Reg1.Bmh, a type I protein phosphatase complex (Sanz et al. 2000; Dombek et al. 2004); three targeting subunits (Schmidt and McCartney 2000); and three upstream kinases (Hong et al. 2003). Many of the genes whose expression is Snf1-dependent encode regulatory proteins, such as protein kinases, protein phosphatases, and transcription factors, suggesting that Snf1 acts through a complex regulatory cascade (Young et al. 2003).
Adr1 and Cat8 are two transcription factors that act downstream of Snf1 to activate nonfermentative metabolic pathways (Schuller 2003). Adr1 and Cat8 act both independently and synergistically to regulate >100 genes after the diauxic transition (Young et al. 2003; Tachibana et al. 2005). One of the Snf1-dependent genes activated by Adr1 and Cat8 is ADH2, encoding alcohol dehydrogenase II, the isozyme that catalyzes the first step in ethanol oxidation. No DNA-binding repressors of ADH2 transcription have been identified (Irani et al. 1987). Instead, ADH2 expression is repressed by the absence of active Snf1, which is kept in an inactive state in the presence of glucose by an active Glc7.Reg1.Bmh complex (Dombek et al. 1993, 2004). Activation (derepression) of ADH2 expression requires the cooperative binding of Adr1 and Cat8, leading to synergistic activation when both factors are present (Walther and Schuller 2001; Tachibana et al. 2005). Snf1 regulates both the expression and the activity of Cat8 (Rahner et al. 1999; Charbon et al. 2004).
Snf1 may regulate Adr1 activity at more than one level as well. Adr1 binding to chromatin is regulated by Snf1 (Young et al. 2002), perhaps through modification of chromatin since Adr1 can bind constitutively to UAS1 in the ADH2 promoter if two histone deacetylases, Rpd3 and Hda1, are absent (Verdone et al. 2002). However, full transcriptional activation of ADH2 does not occur even when Adr1 is bound to the promoter because TBP is not recruited, suggesting a second glucose-regulated step in ADH2 transcription.
The original genetic selection that was used to identify genes that cause constitutive ADH2 expression yielded semi-dominant ADR1c alleles (mutations in a cAPK phosphorylation motif; Cherry et al. 1989) and ADH2 promoter mutations (Ciriacy 1976, 1979; Williamson et al. 1981; Russell et al. 1983). Both classes of mutation act independently of glucose repression. Subsequently, ADR1-independent (CRE1/SPT10/SUD1, CRE2/SPT6/SSN20; Denis 1984), partially ADR1-independent (ADR7, ADR8, and ADR9; Karnitz et al. 1992), and strictly ADR1-dependent recessive constitutive ADH2 mutants (REG1) were identified (Dombek et al. 1993, 1999).
Mutations in REG1 and GLC7 allow glucose-insensitive ADH2 expression only in the presence of ADR1 and SNF1, suggesting that constitutive ADH2 expression in the absence of an active PP1 complex requires the same components that are used normally during derepression (Dombek et al. 1993, 1999). Since loss of either REG1 or GLC7 causes only partial release from repression it is likely that other genes are involved in regulation of ADH2 expression in the presence of glucose. However, since both Adr1 and Cat8 are regulated at both the transcriptional and the post-translational levels (Denis and Gallo 1986; Blumberg et al. 1987; Rahner et al. 1996; Sloan et al. 1999), mutations in a single gene in the repression pathway might not cause constitutive ADH2 expression. This interpretation is supported by two observations. First, mutations in REG1 cause an elevation in both ADR1 expression and activity (Dombek et al. 1993). Second, mutations in GLC7 cause constitutive ADH2 expression only when ADR1 is modestly overexpressed (Dombek et al. 1999). These observations suggest that additional levels of control over repression act directly upon Adr1 or its expression. Although the level of Adr1 protein increases during derepression, elevated Adr1 levels alone are insufficient for full ADH2 activation (Dombek and Young 1997; Sloan et al. 1999).
To identify additional genes required for repression of ADH2 expression, we used a strain containing four copies of ADR1 and an ADH2/lacZ reporter gene. In this strain glucose repression of ADH2 expression is maintained even though Adr1 protein levels in repressed cells are the same as in derepressed cells (Sloan et al. 1999). We assumed that overproduction of Adr1 might overcome the influence of transcriptional repression of ADR1 expression on ADH2 activation and allow us to identify new genes involved in the regulation of Adr1 activity. By screening for constitutive ADH2/β-galactosidase activity we isolated over 100 mutants representing at least 12 complementation groups. Mutants in several of these complementation groups had additional phenotypes, including temperature sensitivity, invertase constitutivity, and abnormal cell morphology. We cloned MOT1, FAB1, VPS35, and CDC10 by genetic complementation of the mutant defects. These genes have known functions in other pathways but had not previously been shown to be involved in glucose repression of gene expression.
MATERIALS AND METHODS
Yeast strains and plasmids:
The S. cerevisiae strains used in this study are listed in Table 1. Multicopy ADR1 strain VBY20 was obtained by integrating an ADH2/GFP reporter into the URA3 locus of JSY24 and crossing a transformant with W303-1B. After sporulation and tetrad dissection a spore with the desired genotype was obtained. Strains designated VMY in the text contain mutations that cause glucose-insensitive ADH2 expression and are derived from either JSY24 (series denoted with “a” following the mutant isolation number) or VBY20 (series denoted with “α” following the mutant isolation number). The double mutants vps35Δsnf1Δ∷kanmx, cdc10-117asnf1Δ∷kanmx, and adr1Δ∷kanmx VMYx shown in Figure 7 and supplemental Figure 1 (http://www.genetics.org/supplemental/) were the products of random spore analysis from diploids made from crossing NKY89α × VMY48a (vps35), NKY89α × VMY117a (cdc10), and NKY66α × VMYa mutants. The random spores were not saved. Knockouts were made using a kanamycin or nourseothricin deletion cassettes (Guldener et al. 1996). The oligonucleotide sequences used are listed in supplemental Table 1 at http://www.genetics.org/supplemental/.
TABLE 1.
Saccharomyces cerevisiae strains
| Strains | Genotype | Source |
|---|---|---|
| JSY24 | MATaadh3 ura3 leu2∷(pRS315-ADR1(LEU2))X3 ADH2∷YIpADH2/lacZ (TRP1) | Sloan et al. (1999) |
| JSY21 | MATaadh3 ura3 leu2∷pRS315-ADR1 (LEU2) ADH2∷YIpADH2/lacZ (TRP1) | Sloan et al. (1999) |
| JSY20 | MATaadh3 ura3 adr1Δ1∷LEU2 ADH2∷YIpADH2/lacZ(TRP1) | Sloan et al. (1999) |
| W303-1B | MATα ade2 cam1-100 his3-11,15 leu2-13, 112 trp1-1 ura3-1 | Yeast stock center |
| NKY66 | W303-1B with Δadr1∷kanmx ADH2∷YIpADH2/lacZ (TRP1) | This work |
| VBY20 | MATα adh3 ura3 his3 leu2∷(pRS315-ADR1)X3 ADH2∷YIpADH2/lacZ(TRP1)ADH2∷ YIpADH2/GFP (URA3) | This work |
| KT1640 | MATα glc7-127 his3 leu2 ura3 | K. Tatchell |
| KDY87 | MATα his3 reg1Δ∷LEU2 ura3 trp1 | K. Dombek |
| SSH35 | MATα adh1 adh3 adr1Δ1∷LEU2 trp1 leu2 ura3 | Karnitz et al. (1992) |
| LK13 | SSH35 with ADH2-10 | This work |
| LK13ΔS | LK13 with snf1∷URA3 | This work |
| S54 | SSH35 with adr7-1 | Karnitz et al. (1992) |
| S54ΔS | S54 with snf1∷URA3 | Karnitz et al. (1992) |
| LK14 | S54 with ADH2-10 | This work |
| LK14ΔS | LK14 with snf1∷URA3 | This work |
| CTY-TY10 | JSY24 with FAB1-HA3-kanmx | This work |
| DYY2 (931) | VBY20 Δpep4∷nat1 | This work |
| DYY4 (933) | VBY20 Δshs1∷nat1 | This work |
| DYY7 (936) | VBY20 Δvac7∷nat1 | This work |
| DYY14 (943) | VBY20 Δvps34∷nat1 | This work |
| DYY16 (945) | JSY24 Δsnf1∷kanmx | This work |
| DYY 18 (947) | JSY24 Δelm1∷nat1 | This work |
| DYY19 (948) | VBY20 Δelm1∷nat1 | This work |
| DYY24 (953) | VBY20 Δreg1∷nat1 | This work |
| DYY28 (957) | VBY20 Δcla4∷nat1 | This work |
| DYY31 (959) | VBY20 Δ420-Celm1∷kanmx | This work |
| NKY75 | VBY20 Δcdc10∷nat1 | This work |
| NKY76 | VBY20 Δfab1∷nat1 | This work |
| NKY89 | VBY20 Δsnf1∷nat1 | This work |
| NKY99 | JSY24 Δsnf1∷kanmx Δreg1∷nat1 | This work |
| NKY77 | VBY20 Δvps35∷nat1 | This work |
| EAY9 | MATa Δfab1∷nat1 Δsnf1∷kanmx HIS3 (other markers the same as VBY20) | This work |
| EAY3 (994) | MATa Δvps34∷nat1 Δsnf1∷kanmx (other markers the same as VBY20) | This work |
Figure 7.
SNF1-independence of constitutive ADH2/lacZ expression. Cells were serially diluted and spotted or patched onto X-gal-8% glucose plates and incubated at 30° or the permissive temperature for ts strains. The strains used are described in the text and materials and methods. Only one dilution is shown.
Yeast genomic libraries of plasmids based on YCp50 or pRS316 (CEN4-URA3) were used for cloning by complementation of function. Yeast plasmids containing septin genes were provided by J. Pringle and the FAB1 kinase-dead plasmid was provided by S. Emr.
Growth of yeast cultures:
Yeast strains were grown in YPD or synthetic medium prepared according to standard methods (Guthrie and Fink 2002). For maintaining glucose repression YP medium contained 8% glucose (YP-high-glucose) and cell density was kept under OD600 = 0.8. X-gal-8% glucose plates used to visualize constitutive expression of ADH2/lacZ were prepared from synthetic medium containing 40 μg/ml 5-bromo-4-chloro-3-idolyl-beta-d-galactopyranoside (X-gal), pH 7.0 (Guarente 1983). Plates containing 5′-fluoro-orotic (5′-FOA) acid were prepared as described (Boeke et al. 1987) and contained 0.1 mg/ml 5′-FOA. Plates containing 1 μg/ml antimycin A were made to select for adh1 mutant cells that have an ADH2-constitutive, antimycin A (At)-resistant phenotype (Williamson et al. 1980).
Mutant isolation:
Strains JSY20, JSY21, JSY24, and VBY20 were treated with 3% ethyl methanesulfonate (EMS) to give 50–60% survival. Cells were plated and incubated at 30° or 25° for 2–3 days and then replica plated onto X-gal-8% glucose plates. Blue colonies on X-gal-high-glucose plates, indicating constitutive activity of an ADH2/lacZ reporter gene under conditions that are repressive for ADH2 expression, were picked, restreaked, and retested. Approximately 60,000, 60,000, 50,000, and 30,000 colonies derived from JSY24, VBY20, JSY20, and JSY21, respectively, were tested. The mutant strains derived from strain JSY24 are designated VMYna, where n is the mutant isolation number and a refers to MATa. Similarly, VMYnα strains are derived from VBY20.
Genetic analysis of mutants:
Complementation tests were performed by pairwise matings of all mutants. Diploids were selected by their Ura+His+ prototrophy and complementation was assessed by colony color on X-gal-8% glucose plates. Representatives from each of the complementation groups were crossed to congenic strains of the opposite mating type to determine whether the constitutive phenotype was due to a single mutation. After sporulation and tetrad dissection spore colonies were replica-plated onto X-gal-8% glucose plates and segregation of the blue/white color among spore colonies from a tetrad was determined.
Cloning:
ADR22 (complementation group XII)—FAB1:
Strain VMY115a (ts, blue on X-gal-8% glucose) was transformed with a yeast genomic library based on pRS316 (CEN4/URA3) at 30°. Ura+ transformants were replica plated onto YP-8% glucose for growth at 35° and onto X-gal-8% glucose plates at 30°. Twenty-five white, temperature-resistant colonies were streaked onto YPD before further analysis. Selected candidates were streaked from the YPD plates onto medium containing 5′-FOA. Twenty-two candidates that gave some 5′-FOA-resistant colonies, indicating the presence of a nonintegrated Ura+ plasmid, were examined further at 35° and on X-gal-8% glucose plates. The library plasmids were recovered from the candidates that exhibited plasmid-dependent complementation of the mutant phenotype, and their ability to complement the original mutant strain was confirmed by retransformation with the purified plasmid. The same plasmids also complemented strain VMY89α, another mutant in complementation group ADR22. Restriction analysis revealed that the complementing plasmids were of three types, designated 115pI, 115pII, and 115pIII. The plasmid inserts were sequenced using the primers YCP50-B1 and YCP50-B2 in the University of Washington Sequencing Facility. The common portion of these three overlapping genomic regions has only one ORF, the FAB1 gene. Plasmids 115pII and 115pIII differ only in the orientation of the genomic fragment present in the vector. The genomic fragment in these plasmids contains a 5′-truncated fragment of the FAB1 gene. A similar fragment complements an FAB1 deletion mutant (Yamamoto et al. 1995). 115pI contained an intact FAB1 gene. Since the only genomic region in common in these three plasmids contains FAB1, or a functional version of FAB1, it is likely that FAB1 corresponds to ADR22. This interpretation was confirmed as described in results.
ADR11 (complementation group I)—VPS35:
A gene representing complementation group I (ADR11) was cloned by complementing the blue-color phenotype on X-gal-8% plates using a yeast genomic library carried on a CEN plasmid. About 8000 Ura+ transformants of strains VMY33a and VMY48a were selected from each transformation and replica plated onto X-gal-8% glucose plates. Colonies that were white, indicating complementation, were restreaked. Candidate colonies were selected for further study if they were blue after losing the library plasmid by selection on FOA plates.
Six of eight independently isolated plasmids from positive transformants of VMY33a had identical restriction maps with three restriction enzymes and two had slightly different maps. The two types of plasmids were sequenced from both sides of the insert. The majority of plasmids contained a region of chromosome X that included two intact ORFs, VPS35 and INO1. A second class of complementing plasmids contained almost the same chromosomal region but the 5′ end of the INO1 gene was missing. Candidate transformants of VMY48a strain yielded two library plasmids. When sequenced, they appeared to contain the same chromosomal region as the majority of complementing plasmids from the transformation of VMY33a, including the VPS35 and INO1 genes. The identification of the mutation in VMY48a was determined by sequencing the entire gene using primers listed in supplemental Table 1 (http://www.genetics.org/supplemental/).
ADR18 (complementation group VIII)—CDC10:
Strain VMY117a was transformed with a yeast genomic library based on pRS316. Approximately 20,000 transformants were plated on SD-ura plates, allowed to recover at room temperature overnight, and then transferred to 37°. After 2 days of incubation four temperature-resistant colonies were isolated. The tsR, Ura+ transformants were β-galactosidase negative, indicating that the constitutive ADH2/lacZ expression in the parent was complemented by the library plasmids. The abnormal cell morphology of VMY117a, long chains of cells with incomplete cytokinesis, was completely suppressed by the library plasmids. Restriction analyses suggested that each transformant contained one or the other of two types of plasmids with overlapping genomic fragments. Retransformation confirmed that the temperature-resistant phenotype and reduced β-galactosidase activity were plasmid dependent. Sequencing with T3 and T7 primers revealed that both types of plasmids contained DNA fragments derived from chromosome III (nucleotides 109224–121326). Only one gene in this region, CDC10, has mutant phenotypes of temperature sensitivity and incomplete cytokinesis that were observed in strain VMY117a (Hartwell 1971; Giaever et al. 2002). CDC10 encodes one of the yeast septins, a family of proteins that form 10-nm fibers involved in cytokinesis-related functions. Nucleotides 109224–116148 of the insert in the library plasmid pLTR(117) were removed by digestion with XbaI to create plasmid pLTR(117)X (containing nucleotides 116149–121326, which includes all of CDC10). Transformation of strain VMY117a with pLTR(117)X allowed growth at 37° and completely suppressed the abnormal cell morphology. The CDC10 allele in strain VMY117a was sequenced on both strands and a single G-to-A base-pair substitution was found at position 131 in the ORF confirming that ADR18 is allelic to CDC10.
ADR7 (mutant13a)—(MOT1):
Mutant 13a failed to complement the temperature sensitivity of an adr7 mutant isolated in an earlier screen (Karnitz et al., 1992). To clone the adr7 mutant, ∼30,000 colonies of strain S54 (adh1 adr7-1) transformed with a yeast genomic library based on Ycp50 were screened for growth at 37°. Temperature-resistant colonies were tested for complementation of a second mutant phenotype, resistance to the respiratory inhibitor Antimycin A (At). The AtR phenotype is a consequence of constitutive expression of ADH2 in strain S54 (Karnitz et al. 1992). Seven of the temperature-resistant colonies were also AtS and thus contained potential ADR7-complementing plasmids. Plasmids from the two AtS Ts+ colonies complemented the adr7-1 mutant phenotype (ts and AtR) of strain S54. Sequencing revealed that they contained the same 14-kbp chromosome XVI fragment. Deletion of an internal 3.6-kbp BamHI fragment eliminated the complementing activity of the plasmid. Subsequent subcloning and sequencing of that region revealed that it contains the MOT1 gene. When strain VMY13a was transformed with the cloned library plasmid containing MOT1, both the ts and ADH2/lacZ constitutive phenotypes were complemented by the MOT1 library plasmid, suggesting that the adr7-1 mutation and the mutation in strain VMY13a are allelic (V. Voronkova, unpublished data).
To test if MOT1 and ADR7 are allelic, the adr7-1 mutation was mapped to the MOT1 locus by integrative transformation and subsequent genetic analysis. A 5′ portion of MOT1 including the promoter but lacking the essential helicase domain was subcloned into pRS306, an integrative URA3 vector, to produce pRS306-SSII. Strain S54 (adr7-1) was transformed with pRS306-SSII linearized by NheI digestion. The resulting strain was crossed to SSH46, a MOT1 wild-type strain. Diploids were selected and sporulated. The phenotypes of the resulting spores were analyzed (Ura+/Ura−, AtR/AtS, growth at 37°). In 18 full tetrads, the Ura+ phenotype always cosegregated with the adr7 phenotype, indicating that ADR7 and MOT1 are allelic or very closely linked (V. Voronkova, unpublished data).
Enzyme assays:
β-Galactosidase assays were performed on permeabilized cells (Guarente 1983). Activities are expressed in Miller units. Secreted invertase activity was assayed in whole cells (Jiang and Carlson 1997). ADH assays were performed on whole cell extracts and by in situ staining of polyacrylamide gels (Dombek et al. 1993).
Protein extracts and Western blotting:
Denatured whole-cell extracts were prepared as described previously (Dombek et al. 1993) or as in Kushnirov (2000). Proteins were separated by SDS–PAGE, transferred to a nylon membrane, and analyzed by immunoblotting using polyclonal rabbit antibodies raised against amino acids 335–740 of Adr1 and IR-labeled secondary antibodies (Rockland). Visualization and quantitation were performed on an Odyssey infrared imaging system (Li-Cor Biosciences) according to manufacturer's directions.
RESULTS
Constitutive ADH2 expression in ADR1 multicopy strains lacking REG1 or Glc7 protein phosphatase activity:
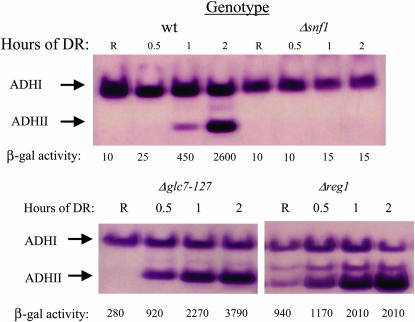
In preparation for using multicopy ADR1 strains in a genetic screen for constitutive ADH2 expression, we tested their ability to detect known mutations in ADH2 regulation by deleting REG1 and SNF1 in VBY20. GLC7 was deleted in the presence of a ts allele of GLC7 carried on a plasmid (Dombek et al. 1993, 1999). The multicopy strains reveal the constitutive phenotypes of the reg1 and glc7 mutants by both an in-gel activity assay for endogenous ADH2 expression and by an ADH2/lacZ reporter assay. Snf1 is required for derepression in the multicopy ADR1 strain (Figure 1) and for constitutive expression in the multicopy ADR1, reg1-deletion strain (N. Kacherovsky, unpublished data). Thus, the multicopy ADR1 strain is an appropriate starting point for screening for regulatory mutations affecting ADH2 repression.
Figure 1.
Constitutive ADH2 and ADH2/lacZ expression in multicopy ADR1 strains with mutations in regulatory genes. In-gel ADH activity assays of extracts prepared from strain JSY24, Δsnf1, Δreg1, and Δglc7 (YCpGLC7-127) were performed as described in materials and methods. R indicates extracts prepared from repressed cultures grown at 30°. The other time points represent hours after shifting the cells to derepressing (DR) medium (YP with 0.05% glucose). The numbers below each lane represent the β-galactosidase activities in Miller units. The band between ADHI and ADHII also represents ADHII expression, since it is a heterodimer of the two enzymes.
Mutant screen:
Mutagenesis of JSY24 (MATa) and VBY20 (MATα), both of which contain three extra copies of ADR1 integrated at the LEU2 locus, yielded 191 mutants that exhibited constitutive expression of the integrated ADH2/lacZ reporter. Strains with zero (JSY20) or one (JSY21) copy of ADR1 yielded one and two mutants, respectively, when the same number of surviving cells was plated. The dramatic difference in the number of mutants isolated from strains with zero (or one) and three integrated copies of ADR1 confirms the importance of performing the mutant isolation in a strain that expresses Adr1 at derepressed levels. Presumably, the additional amount of Adr1 magnifies the effect of a mutation that allows expression from the ADH2 promoter under repressed conditions. Thus, by satisfying the requirement for the positive activator, we can more readily identify genes that play a negative role in ADH2 expression. The constitutive ADH2/lacZ expression in the mutant strains could be due either to cis-acting mutations located in the promoter region of the ADH2/lacZ reporter gene or to trans-acting mutations that affect its expression. Expression of the chromosomal ADH2 locus was assayed to distinguish between these possibilities since only trans-acting mutations would affect its expression. The majority of the mutants exhibited ADHII activity after growth under repressed conditions, as revealed by in-gel activity assays (V. Voronkonva, unpublished data). The mutants that did not show detectable ADHII activity were not studied further (∼10% of mutants). Thus, most of the mutations conferring constitutive activity on an ADH2/lacZ reporter gene also cause constitutive expression of the chromosomal ADH2 locus and are therefore trans-acting.
Genetic analysis:
To determine whether the mutants are recessive or dominant they were backcrossed to a congenic wild-type strain and the constitutive ADH2/lacZ phenotype was determined. Most of the diploids were white, indicating a recessive mutation, but several of them were blue (dominant). Among 101 MATa mutants 93 are recessive and 8 are dominant. Among 90 MATα mutants 79 are recessive and 11 are dominant.
Complementation tests were performed by pairwise matings of all recessive mutants. The diploids were selected and examined for ADH2/lacZ constitutive activity on X-gal-8% glucose plates. One-third of the MATa and most of the MATα mutants belong to 1 of 12 complementation groups, I–XII (Table 2). We named the 12 genes represented by these complementation groups ADR11–ADR22 (alcohol dehydrogenase regulator 11–22). The mutants that do not fall into any of the 12 complementation groups could harbor multiple mutations, represent additional genes affecting ADH2 repression, or harbor weak alleles that fail to exhibit a constitutive ADH2 phenotype in a diploid strain. All mutants were also tested for complementation of mutations in REG1, GLC7, ADR7, ADR8, and ADR9 since mutations in these genes cause constitutive ADH2 expression (Karnitz et al. 1992; Dombek et al. 1993, 1999). VMY13a and VMY78a, which did not belong to one of the complementation groups containing multiple alleles, failed to complement strains containing mutations in ADR7 and ADR8, respectively, suggesting that they contain mutant alleles of those genes.
TABLE 2.
Genetic and phenotypic properties of new ADR mutants
| Allele
|
||||
|---|---|---|---|---|
| Complementation group | Gene | MATa | MATα | Additional phenotypes |
| I | ADR11 | 33,a 38, 48,ab 109, 114,a 37ab | 1, 30, 32, 62 | Elevated ADH2 Expression at 25°C |
| II | ADR12 | 17a | 5, 9, 59, 89ab | Abn |
| III | ADR13 | 70, 112 | 19, 35, 85 | Spo− |
| IV | ADR14 | 103, 119a | 6, 10 | Abn |
| V | ADR15 | 3,b 15 | 52 | Spo− |
| VI | ADR16 | 3,b 63,a 101a | 25 | |
| VII | ADR17 | 110 | 20, 66 | Spo− InvC |
| VIII | ADR18 | 53, 117,a 141 | 49 | ts, hs, Abn InvC |
| IX | ADR19 | 37,ab 59, 127, 128 | 44 | ts, hs, InvC |
| X | ADR20 | 16, 43 | 97, 102 | |
| XI | ADR21 | 44, 48,ab 105, 115ab | 79 | ts |
| XII | ADR22 | 115ab | 89ab | ts |
Additional mutants that did not fall into any of the complementation groups are not shown and were not studied further except for two that failed to complement known genes. They are mutant 13a, a temperature-sensitive mutant that failed to complement mutations in ADR7, and mutant 78a, which failed to complement mutations in ADR8.
Spo−, sporulation deficiency in heterozygous diploids; Abn, abnormal cell morphology; InvC, constitutive invertase activity; ts, temperature sensitive; hs, heat-shock sensitive.
Phenotype is due to a single gene.
Shows nonallelic noncomplementation. That is, it complements mutants in more than one complementation group. For example, VMY89α fails to complement mutants in both complementation group II and complementation group XII. In the case of VMY89α and VMY115a, the true complementation group is XII; for VMY48a, the true complementation group is I; and for VMY37a, the true complementation group is IX, as described in the text. The true complementation group for VMY3a was not determined.
Several cases of nonallelic noncomplementation were observed, suggesting that some complementation groups contain mutant alleles from different genes. For example, mutant VMY37a fails to complement mutants VMY62α and VMY1α in complementation group I and also fails to complement mutant VMY44α in complementation group IX. To determine the true complementation group for mutant VMY37a, diploids derived from crosses of VMY37a with VMY62α and with VMY44α were subjected to tetrad analysis. If the two parents harbor a mutation in the same gene, then all four spores will inherit a mutation in the same gene and will have a mutant phenotype. A 4:0 segregation of blue/white spores was observed for diploids derived from crossing VMY37a with a member of complementation group IX, VMY44α, but not from a cross with VMY62α, a mutant from complementation group I. This result indicates that mutant VMY37a belongs to complementation group IX and shows nonallelic noncomplementation with mutants in complementation group I.
Because several members of complementation group I showed aberrant complementation behavior, we confirmed that assignment in the following manner. MATα derivatives of VMY48a (harboring a mutation in complementation group I) and two mutants that do not belong to any complementation group, VMY25a and VMY42a, were obtained from a backcross to the parental strain and were crossed to the whole set of the MATa mutants. The diploids obtained were tested for complementation of the mutant ADH2/lacZ phenotype. The MATα derivative of VMY48a failed to complement all MATa members of complementation group I but complemented all other MATa mutants. The MATα derivatives of VMY25a and VMY42a failed to complement only the original mutants. These results suggest that the observation of nonallelic noncomplementation is not due to incorrect assignment of the mutations to their complementation groups. Although the importance of these observations is unclear, it suggests that the genes represented by these complementation groups carry out functionally related processes affecting ADH2 repression.
Constitutive chromosomal ADH2 expression:
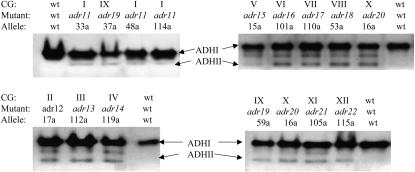
To confirm that the mutations in the defined complementation groups are trans-acting and thus affect the expression of the chromosomal ADH2 gene we performed in-gel ADH activity assays for two mutants in each complementation group. The mutants analyzed each contain a single mutation that affects ADH2/lacZ expression as shown by tetrad analysis. Mutants from each complementation group exhibit different but consistent levels of constitutive ADHII activity (Figure 2).
Figure 2.
Constitutive ADHII activity of representatives from complementation groups I–XII (adr11-adr22). In-gel ADH activity assays were performed as described in materials and methods. Cell extracts were prepared from cultures grown in YP-8% glucose to an A600 ∼1. JSY24 is the wild-type parental strain. The band between ADHI and II also represents ADHII expression since it is a heterodimer of the two enzymes. See Table 2 for a complete description of the mutant phenotypes.
Growth phenotypes associated with adr11-adr22 mutants:
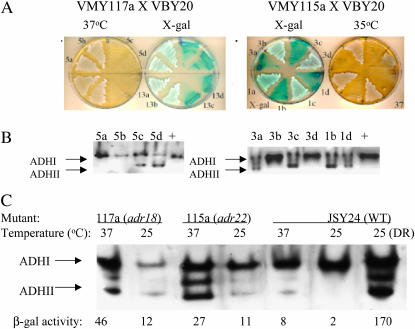
We tested the mutants for heat and cold sensitivity, heat-shock sensitivity, abnormal cell morphology, and enhanced β-galactosidase activity at 25° or 35° (data summarized in Table 2 for mutants in complementation groups I–XII). Five mutants, representing 4 complementation groups (VIII, IX, XI, XII), as well as five mutants not belonging to one of the 12 groups, are sensitive to elevated temperature (ts), but none are cold sensitive. The two ts mutants in complementation groups VIII and IX are heat-shock sensitive as well. ts mutant VMY117a in complementation group VIII showed a pronounced cytokinesis defect even at the permissive growth temperature. All members of complementation group I displayed enhanced blue color at 25° on 8% glucose X-gal plates compared to wild type, suggesting a cold-sensitive step in ADH2 repression in these mutants. The ts mutants are particularly interesting because they indicate that an essential gene plays a role in repressing ADH2 expression. To determine whether the ts and the constitutive ADH2 phenotypes are due to the same mutation, the strains bearing ts alleles were back-crossed to the appropriate parental strain. The heterozygous diploids were sporulated and the distribution of phenotypes among spore colonies was analyzed. The constitutive β-galactosidase activity, ADHII activity on glucose measured by in-gel assay, and temperature sensitivity segregated 2:2 and cosegregated with each other for mutants VMY115a and VMY117a (Figure 3, A and B). Mutants VMY89α and VMY111a behaved similarly (V. Voronkova, unpublished data). Thus, these four strains harbor mutations in a single gene that is essential and is involved in glucose repression of ADH2 expression. A similar analysis was performed on 12 other mutants. In six cases the constitutive phenotype appeared to be due to more than one mutation, suggesting that some of the mutants harbor multiple lesions that are responsible for their constitutive activity.
Figure 3.
A single mutation causes temperature sensitivity and constitutive ADH2 expression in mutants VMY115a and VMY117a. Spore colonies from dissected tetrads arising from a cross of the mutant strain VMY117a (tetrads 5 and 13) and VMY115a (tetrads 1 and 3) with VBY20 were tested for β-galactosidase activity on X-gal-8% glucose plates and for temperature sensitivity (A). ADH activity was assayed using an in-gel assay of selected spore colonies or wild type (+ lane) (B). Temperature sensitivity of ADH2 expression in VMY117a and VMY115a was assayed by in-gel assays of ADH activity and ADH2/lacZ expression at 25° and 2 hr after a shift to 37°. The ADH and β-galactosidase activity assays were performed on repressed cultures. JSY24 is the parental strain for VMY115a and VMY117a.
If a single mutant gene is responsible for impaired ADH2 glucose repression and ts growth phenotype, then at the nonpermissive temperature the mutant protein should not only fail to support growth but also fail to sustain ADH2 repression. To test this we assayed ADH2 constitutive activity at the nonpermissive temperature. Mutants were grown at the permissive (25°) temperature and then shifted to 37°. Cells were collected and β-galactosidase and ADH II activities were determined. Figure 3C shows that at the nonpermissive temperature mutants VMY115a and VMY117a exhibited increased β-galactosidase and ADHII activities, consistent with the interpretation that a single mutation is responsible for both phenotypes.
Invertase and ADH2 expression in adr mutants:
We measured secreted invertase activity of two members of each complementation group after growth on glucose to determine if the mutants affect other glucose-repressed genes. Strains containing mutations in three complementation groups, ADR17, ADR18, and ADR19, have elevated levels of secreted invertase activity when grown under repressed conditions (Table 3). The highest invertase activity is exhibited by strains containing either one of two temperature-sensitive mutations, 117a (adr18, 8-fold wild type) or 37a (adr19, 11-fold wild type).
TABLE 3.
Invertase and ADH2/β-galactosidase activities in adr mutant strains
| Invertase activity
|
β-Galactosidase activity
|
||||
|---|---|---|---|---|---|
| Gene | Alleles | R | DR | R | DR |
| ADR11 | 33a | 2 | 260 | 13 | 320 |
| 114a | 3 | 260 | 11 | 350 | |
| ADR12 | 17a | 8 | 270 | 16 | 310 |
| ADR13 | 70a | 3 | 220 | 20 | 270 |
| 112a | 5 | 240 | 28 | 330 | |
| ADR14 | 119a | 2 | 250 | 18 | 300 |
| 103a | 5 | 230 | 16 | 280 | |
| ADR15 | 3a | 3 | 250 | 15 | 330 |
| 15a | 5 | 280 | 12 | 350 | |
| ADR16 | 63a | 8 | 250 | 16 | 320 |
| 101a | 6 | 280 | 18 | 350 | |
| ADR17 | 110a | 12 | 270 | 14 | 360 |
| ADR18 | 53a | 10 | 210 | 24 | 300 |
| 117a | 24 | 400 | 20 | 360 | |
| ADR19 | 37a | 35 | 400 | 26 | 360 |
| 127a | 12 | 280 | 18 | 320 | |
| ADR20 | 16a | 6 | 280 | 14 | 320 |
| 43a | 3 | 250 | 14 | 300 | |
| ADR21 | 105a | 4 | 250 | 14 | 300 |
| 44a | 6 | 270 | 15 | 350 | |
| ADR22 | 115a | 4 | 320 | 19 | 370 |
| Wild type (JSY24) | 3 | 280 | 2 | 350 | |
Enzyme activities were determined as described in materials and methods. The values represent the average of three determinations. The standard deviation for each value was ∼10%.
R, repressed; DR, derepressed.
β-Galactosidase activity of the same mutants was determined in the same preparations to have a quantitative measure of constitutive expression of the ADH2/lacZ reporter (Table 3). The constitutive activity in the mutant cells is 5–14 times higher than the activity under repressed conditions of wild-type cells. Under derepressed conditions the β-galactosidase activities are close to that of the wild-type strain as measured by an endpoint assay. The constitutive activities in the mutant strains represent 3–10% of the activity after derepression, significantly lower than the activity present in the absence of Reg1 (Figure 1) suggesting that these mutations and a reg1 deletion do not disrupt the same pathway of repression.
ADR1-dependence of constitutive ADH2 expression:
The large number of constitutive mutants isolated in the multicopy ADR1 strains compared to strains with a single copy of ADR1 suggests that the constitutive ADH2 expression is ADR1 dependent. This prediction was tested by analyzing spore colonies grown from tetrads derived from crosses of one mutant from each complementation group to a congenic wild-type strain of the opposite mating type in which ADR1 had been deleted and replaced by a kanMX cassette. The different alleles of ADR1 could be identified from their phenotypes on plates (adr1 null: Leu−, KanR; single copy: KanS, Leu−; or multicopy: Leu+, KanS, or KanR). Constitutive ADH2/lacZ expression was monitored on X-gal plates. Because the mutation could not be followed directly, its presence or absence was inferred from the color on indicator plates. Thus, if all ADR1-null (Leu− KanR) spore colonies were white or light blue on X-gal plates, and some multicopy (Leu+) ADR1 spore colonies were dark blue, the phenotype was judged to be ADR1 dependent. The level of Adr1 in suspected mutant spore colonies was confirmed by Western blotting for Adr1. On the basis of these criteria, the constitutive ADH2 expression in all of the mutants is ADR1 dependent, although some, such as VMY89α, showed a stronger dependence on ADR1 copy number than others, such as VMY49α (D. Yu, unpublished data, and supplemental Figure 1 at http://www.genetics.org/supplemental/).
Cloning and identifying ADR11, ADR18, ADR22, and ADR7:
Complementation group XII—ADR22 (FAB1):
Complementation group XII (ADR22) consists of two ts mutants, 115a and 89α, that exhibit impaired glucose repression of ADH2 but not SUC2 expression. Thus, ADR22 is an essential gene that is involved in a pathway of glucose repression that affects ADH2 but not SUC2 expression. This complementation group is also interesting because both 115a and 89α exhibit nonallelic noncomplementation with mutants in other complementation groups (Table 2).
ADR22 was cloned by complementing the constitutive ADH2 expression and ts mutant phenotypes using a low-copy yeast genomic library (materials and methods). The common portion of the cloned genomic regions have only one complete or nearly complete ORF and it encodes the FAB1 gene encoding 1-phosphatidylinositol-3′-phosphate-5′-kinase [PtdIns (3′)-P-(5′)-kinase] (Yamamoto et al. 1995; Bonangelino et al. 2002; Gary et al. 2002).
The cloned FAB1 gene complemented all mutant phenotypes of VMY89α and VMY115a, suggesting that FAB1 corresponds to ADR22. An enlarged vacuole is a phenotype previously noted for conditional FAB1 mutants (Yamamoto et al. 1995). The greatly enlarged single vacuole in the 115a and 89α mutants is restored to wild-type size and shape by FAB1-containing plasmids (V. Voronkova, unpublished data). A deletion of FAB1 has a ts phenotype (Yamamoto et al. 1995) consistent with the phenotype of strains VMY115a and VMY89α and suggesting that these mutations represent the null FAB1 phenotype.
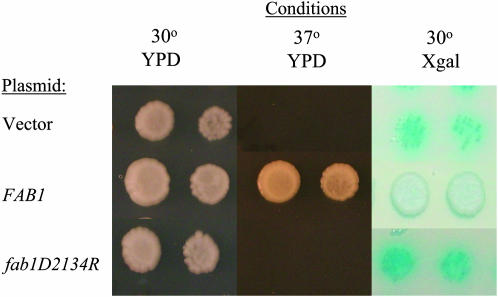
A FAB1 mutation in VMY115a was confirmed in three ways. First, when a diploid VMY115a fab1ts/FAB1:kanMX-tag strain was sporulated, nine of nine full tetrads segregated 2:2 for G418R:ts (C. Tachibana, unpublished data). Since the ts character and ADH2 constitutivity are genetically linked (Figure 3), strains VMY115a and VMY89α most likely contain mutant alleles of FAB1. Second, the entire FAB1 ORF was sequenced in strains VMY115a and VMY89α. A nonsense mutation, CAA to TAA, corresponding to Gln1686, is present in both strains causing loss of the C-terminal kinase domain. This mutation is sufficient to explain the temperature sensitivity of strains VMY115a and VMY89α since a kinase-dead mutant of FAB1 is temperature sensitive (Yamamoto et al. 1995). Third, we tested a kinase-dead mutant for constitutive ADH2/lacZ expression by introducing a plasmid carrying a fab1-kd allele into VBY20Δfab1. The kinase-dead allele, a substitution of Asp2134 by Arg in the kinase catalytic domain, failed to complement the constitutive activity and temperature sensitivity, whereas the wild-type FAB1 gene carried on a plasmid complemented both phenotypes (Figure 4) indicating that Fab1 kinase activity is required to maintain glucose repression of ADH2 expression.
Figure 4.
Repression of ADH2 expression requires the kinase activity of Fab1. VBY20Δfab1 was transformed with URA3-CEN plasmids pRS316, pFAB1(115pI), or pfab1D2134R. Transformants were grown to log-phase in YPD (8% glucose), serially diluted, and spotted onto YPD plates at 30° and 37° and onto X-gal-8% glucose plate at 30°. Two dilutions are shown.
Strains VMY115a and VMY89α (ADR22) failed to complement strains containing mutations in ADR12 and ADR21. These mutants were not complemented with a plasmid containing FAB1, confirming that ADR12 and ADR21 show nonallelic noncomplementation with FAB1.
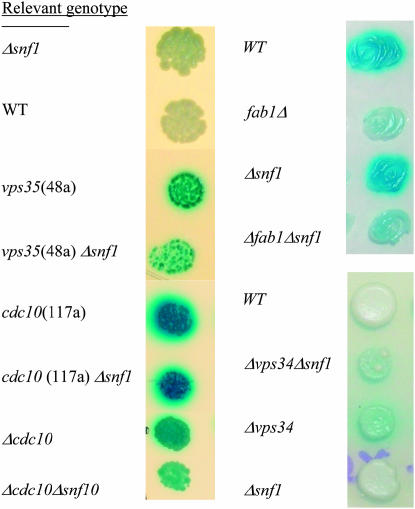
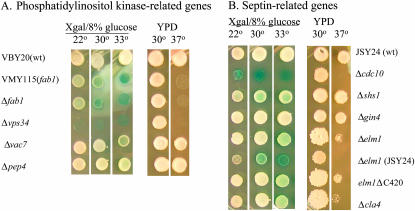
Fab1 phosphorylates PtdIns-3′-phosphate, the product of the reaction catalyzed by the PtdIns-3′-kinase Vps34. If PtdIns-3′,5′-phosphate is important in glucose repression, as suggested by the constitutive activity of ADH2 in a fab1 mutant, constitutive ADH2 expression would be expected in a vps34 mutant strain. Constitutive ADH2 expression was indeed observed in the ADR1 multicopy strain VBY20 with a vps34∷kanMX allele, and, like the fab1 mutants, the strain is temperature sensitive for growth (Figure 5A). The strain with a deletion of VPS34 grew very poorly on the X-gal plates, indicative of a high level of constitutive ADH2/lacZ expression. As with the strain with a mutation in FAB1, the constitutive ADH2/lacZ expression was elevated at 30° and 33° compared to 22°. This result indicates that PtdIns-3′-phosphate is important in glucose repression, presumably as a precursor to PtdIns-3′,5′-phosphate. VAC7 encodes a protein that, like Fab1 and Vps34, is implicated in vacuolar biogenesis (Gary et al. 2002). However, deleting VAC7 did not cause constitutive ADH2/lacZ expression, indicating that Vac7 does not play a role in ADH2 repression (Figure 5A). A defect in general vacuolar function is not the source of constitutive ADH2/lacZ expression because deletion of PEP4, a gene encoding the major processing protease in the vacuole, did not cause constitutive ADH2 expression (Figure 5A). In summary, the results implicate PtdIns-3′,5′-phosphate in maintaining glucose repression of ADH2 expression.
Figure 5.
Constitutive ADH2/lacZ expression in strains with mutations in phosphatidylinositol kinase- and septin-related genes. Growth and reporter tests were conducted by diluting log-phase yeast cultures and spotting serial dilutions onto YPD plates at 30° and 37° and onto X-gal-8% glucose plates that were incubated at 22°, 30°, and 33°. Only one dilution is shown. The strains used are described in the text and Table 1.
Complementation group I—ADR11(VPS35):
Complementation group I (ADR11) is the largest of the 12 complementation groups and all nine members exhibit enhanced ADH2/lacZ expression at 25°. Since ADR11 mutants do not have constitutive invertase activity, they are unlikely to have mutations in genes previously known to affect glucose repression. Mutations in this group also display nonallelic noncomplementation with ts mutant 37a in ADR19.
ADR11 was cloned by complementing the ADH2/lacZ constitutive mutant phenotype of strains VMY33a and VMY48a (materials and methods). The data in materials and methods suggest that VPS35 is the complementing gene on the isolated plasmids. VPS35 was recovered in a genetic screen to isolate mutants deficient in sorting hydrolases from the Golgi to the vacuole (Paravicini et al. 1992). Vps35 is a part of the retromer complex and is responsible for cargo-selective activity (Burda et al. 2002).
The entire VPS35 ORF in all mutants in complementation group I was sequenced. A single missense mutation was found in strain VMY48a, changing Glu433 to Gly in the VPS35 ORF. None of the other mutants contained a mutation in the VPS35 ORF, demonstrating that the Glu433-to-Gly change in VMY48a is a mutation and not a strain-specific polymorphism. Since all of the mutants in this complementation group were complemented by VPS35 carried on the library plasmid (N. Kacherovsky, unpublished data), they could contain non-ORF mutations that affect expression of VPS35, or they could, like VMY37a, represent examples of nonallelic noncomplementation. However, strain VMY37a, which has a mutation in complementation group IX and shows nonallelic noncomplementation with mutants in complementation group I, was not complemented by VPS35 carried on a plasmid.
Complementation group VIII—ADR18 (CDC10):
The mutation in strain VMY117a, in complementation group VIII (ADR18), causes temperature sensitivity, a cytokinesis defect, and constitutive ADH2 and SUC2 expression. Library plasmids complementing the temperature sensitivity of VMY117a contained overlapping fragments that also complemented the constitutive ADH2 expression and incomplete cytokinesis. CDC10 was shown by subcloning to be the ORF responsible for complementation. Mutations in CDC10 as well as a deletion of the CDC10 ORF cause temperature-sensitive growth and incomplete cytokinesis (Hartwell 1971; Frazier et al. 1998) suggesting that VMY117a contains a loss-of-function mutation in CDC10.
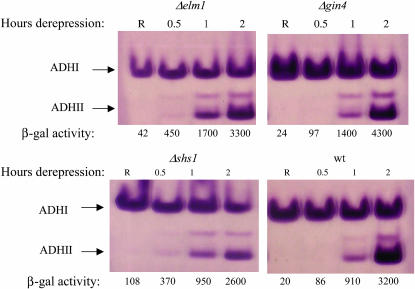
CDC10 encodes one of the yeast septins, a family of proteins that form 10-nm fibers involved in cytokinesis-related functions (Longtine and Bi 2003). Proof that VMY117a is defective in CDC10 was obtained by finding a single missense mutation, GGT to GAT, in CDC10. The mutation creates a Gly44-to-Asp substitution in a GTP-binding motif (Flescher et al. 1993). Mutations in the GTP-binding motif of the homologous yeast septin encoded by CDC11 inhibit GTP binding but have only a modest phenotypic effect (Casamayor and Snyder 2003). The Gly44-to-Asp substitution in Cdc10 could have a more severe defect in GTP binding than related mutations in Cdc11. Alternatively, GTP binding and hydrolysis by Cdc10 may be more important for Cdc10 function than it is for Cdc11. A deletion of the CDC10 locus was created by nat1 (nourseothricin resistance) insertion in the ADR1 multicopy strain VBY20. The resulting strain was ts, had a cytokinesis defect at 30°, and expressed ADH2 constitutively (Figures 5B and 6, and N. Kacherovsky, unpublished data), consistent with the sequencing data indicating that a lesion in CDC10 in strain VMY117a causes constitutive ADH2 expression. Mutations in other nonessential septins (SHS1, SPR3, SPR28) as well as in genes functionally related to the septins (CLA4, GIN4, ELM1, GAC1) were tested for constitutive ADH2/lacZ expression in strain VBY20 after deletion using a nat1 cassette. Strains lacking SHS1, encoding a nonessential septin homolog, and ELM1, encoding a protein kinase involved in septin assembly (Sreenivasan and Kellogg 1999) and also acting as a Snf1-activating kinase (Hong et al. 2003), had weak constitutive ADH2/lacZ expression that was elevated at 30° and 33° relative to 22°. In addition, at 33° constitutive ADH2/lacZ expression could be detected in the strain deleted for CLA4. A strain in which the C-terminal inhibitory domain of Elm1 had been deleted had similar phenotypes. Deletion of SPR3, SPR28, GIN4, and GAC1 did not cause constitutive ADH2/lacZ expression (Figures 5B and 6, and N. Kacherovsky, unpublished data). The constitutive activity of the elm1 deletion allele, as well as temperature sensitivity, was more pronounced in JSY24, another multicopy ADR1 strain (Figure 5B). Constitutive ADH2 expression was also tested in elm1, gin4, or shs1 deletion strains by measuring chromosomal ADH2 expression with an in-gel activity assay and β-galactosidase activity from an integrated reporter. Although constitutive ADH2 expression could not be detected by the in-gel assay, more rapid derepression of both ADH2 and ADH2/lacZ was detected in elmΔ and shs1Δ strains compared to a wild-type strain or a strain with a gin4 deletion. As predicted from the color phenotype of X-gal-8% glucose plates, the constitutive β-galactosidase activity was elevated in the elmΔ and shs1Δ strains (Figure 6). Two other strains in complementation group VIII, VMY53a and VMY49α, failed to be complemented by CDC10 or the septins CDC3, CDC11, CDC12, or SHS1 on a plasmid. Sequencing of the entire CDC10 ORF in these strains did not reveal a mutation (N. Kacherovsky, unpublished data). These strains apparently show nonallelic noncomplementation with a strain with a mutation in CDC10. In summary, alteration of septin function or assembly can result in constitutive ADH2 and SUC2 expression.
Figure 6.
ADH2 expression in septin-related mutants gin4, shs1, and elm1. In-gel ADH activity assays were performed on extracts from strains with mutations that cause septin formation defects. R indicates extracts prepared from repressed cultures grown at 30°. The other time points represent hours after shifting the cells to derepressing medium (YP with 0.05% glucose). The numbers below each lane represent the β-galactosidase activities in Miller units.
SNF1-dependence of constitutive activity in strains containing mutations in VPS35, FAB1, VPS34, or CDC10:
The SNF1 dependence of constitutive ADH2 expression was tested by deleting SNF1 using a kanMX or nat1 cassette in strains VMY48a (VPS35), VMY89α (FAB1), and VMY117a (CDC10). The SNF1 dependence of VPS34 was also tested since it encodes the kinase upstream of FAB1 in the phosphoinositide pathway and also caused constitutive ADH2 expression. For fab1 mutant strains we were unable to recover snf1 deletion transformants so JSY24 Δsnf1∷kanmx was mated to VBY20 Δ fab1∷nat1 or VBY20 Δvps34∷nat1, the heterozygous diploids were sporulated, and haploid double mutants were identified by their resistance to both nourseothricin and G418. In this manner vps34snf1 double mutants were easily recovered. However, the heterozygous FAB1/fab1∷nat1 snf1∷kanmx/SNF1 diploid gave very poor sporulation and no fab1snf1 mutants, possibly due to a germination defect. To overcome this problem, the fab1∷nat1/FAB1 snf1∷kanmx/SNF1 diploid was transformed with a FAB1-URA3-CEN plasmid. The resulting diploid gave better sporulation and many fab1snf1 double mutants, all of which were Ura+, indicating that they contained the FAB1-URA3 plasmid. Selection on 5′-FOA yielded fab1snf1 double mutants lacking the FAB1 gene. The fab1snf1 and vps34snf1 double mutants were temperature sensitive for growth (indicating fab1 or vps34 mutations) and glycerol negative (snf1), yet exhibited constitutive ADH2/lacZ expression. The constitutive ADH2 expression of vps35 and cdc10 mutants was also SNF1-independent (Figure 7). Thus, all these mutations represent alterations in pathway(s) distinct from repression mediated by Glc7.Reg1.Bmh1 and by Mot1 (see below). Mutations in any of these genes cause constitutive ADH2 expression only in the presence of an active SNF1 allele (Dombek et al. 1993, 2004).
ADR7 (mutant 13a)—MOT1:
ts mutant 13a did not fall into 1 of the 12 complementation groups. However, it failed to complement strains containing mutations in ADR7 that were isolated in a previous screen for constitutive ADH2 expression in the presence of an overexpressed, normally inactive ADR1-220 allele (Karnitz et al. 1992). Both ADR7 mutant alleles, like mutant 13a, are temperature sensitive.
ADR7 (mutant 13a) was cloned by complementing the ts and AtR (antimycin A) mutant phenotypes of strain S54 as described in materials and methods. Sequence analysis of the complementing fragment revealed that it contains MOT1. The cloned YCp50-MOT1 library plasmid also complements the VMY13a mutant phenotypes, consistent with genetic analysis showing allelism between ADR7 and mutant13a (V. Voronkova, unpublished data).
Allelism between MOT1 and ADR7 was demonstrated by integrative transformation and subsequent genetic analysis as described in material and methods (V. Voronkova, unpublished data). These results indicated that adr7-1 is closely linked to or within MOT1. Since the transcriptional defects of adr7-1 and mutant 13a are similar to those of other mot1 temperature-sensitive alleles, the mutations are most likely in MOT1 itself.
Mot1 is a general repressor of Pol II transcription and acts by displacing TBP from the TATA box in an ATP-dependent fashion (Auble and Hahn 1993; Auble et al. 1994, 1997). The fact that in two different screens for constitutive ADH2 expression we isolated MOT1 mutants confirms that Mot1 is important for maintaining glucose repression of ADH2 expression.
mot1 (adr7)-dependent constitutive ADH2 activity requires SNF1 and acts independently of the ADH2-10 deletion:
Since mutations in MOT1 are known to cause gene expression that is partially independent of normal activator requirements in vivo (Davis et al. 1992), it is important to know if the constitutive ADH2 expression acts through the normal pathway of derepression, which requires Adr1 and Snf1. Since the effects of adr7-1 are stronger in the presence of ADR1-220, which is an overexpressed, truncated allele of ADR1 that has very low activity in a wild-type strain (Karnitz et al. 1992), we used a strain deleted for the wild-type copy of ADR1 and carrying a plasmid expressing either ADR1-220 or no ADR1. As shown in Table 4 the ADHII activity was low in the parent strain, SSH35, with ADR1-220. Activity increased about sixfold in the adr7-1 mutant in an ADR1-220-dependent fashion (compare lines 1–3).
TABLE 4.
ADHII activities in adr7-1 (mot1) mutant strains
| Genotype
|
|||||||
|---|---|---|---|---|---|---|---|
| Line | Strain | MOT1 | SNF1 | ADR1a | ADH2 | ADHII activity (R)b | AtRc |
| 1 | SSH35 | WT | WT | 220 | WT | 29 | − |
| 2 | S54 | adr7-1 | WT | 220 | WT | 195 | +++ |
| 3 | S54 | adr7-1 | WT | Δ | WT | 50 | −+ |
| 4 | S54ΔS | adr7-1 | Δ | 220 | WT | 20 | − |
| 5 | S54ΔS | adr7-1 | Δ | Δ | WT | 22 | − |
| 6 | LK13 | WT | WT | 220 | ADH2-10 | 240 | +++ |
| 7 | LV14 | adr7-1 | WT | 220 | ADH2-10 | 500 | +++ |
| 8 | LK13ΔS | WT | Δ | 220 | ADH2-10 | 18 | − |
| 9 | LV14ΔS | adr7-1 | Δ | 220 | ADH2-10 | 75 | −+ |
| 10 | LK13ΔS | WT | Δ | Δ | ADH2-10 | 15 | − |
| 11 | LV14ΔS | adr7-1 | Δ | Δ | ADH2-10 | 50 | −+ |
Cells were grown in synthetic medium containing 5% glucose and crude cell extracts were assayed for ADH activity as described in materials and methods.
The ADR1-220 allele was present on a CEN4-TRP1 plasmid (Karnitz et al. 1992). In strains denoted Δ in the ADR1 column the pRS314 vector was present.
The ADH activity is the average of triplicate measurements that had a standard deviation of ∼10%.
Growth in the presence of the respiratory inhibitor Antimycin A (AtR) is indicative of constitutive ADH2 expression (Karnitz et al. 1992). Growth was scored as good growth (+++), very poor growth (−+), or no growth (−) after replica plating patches from a plate containing synthetic Trp− medium supplemented with 5% glucose.
The activity decreased to the wild-type level in the absence of SNF1 (line 4). These data indicate that the constitutive ADH2 expression caused by a mutation in MOT1 requires both ADR1 and SNF1. Even the weak ADR1-independent constitutive expression observed in the adr7-1 mutant is SNF1 dependent (compare lines 3 and 5). Although MOT1 (ADR7) behaves genetically like a repressor of ADH2 expression, it does not have sequence-specific DNA-binding properties and is thus unlikely to bind the ADH2 promoter. Nonetheless, its activity requires DNA upstream of the TATA box (Darst et al. 2001). ADH2-10 is a 12-bp deletion/insertion mutation in the ADH2 promoter, located 40 bp upstream of the TATA box (−104 to −110). ADH2-10 is constitutively active in the presence of the ADR1-220 allele, whereas wild-type ADH2 is not (Karnitz et al. 1992). This phenotype is identical to the phenotype caused by loss of Mot1 activity (Table 4, compare lines 2 and 6). If Mot1 activity at the ADH2 promoter requires the sequence deleted in ADH2-10, then a strain containing both the adr7-1 (mot1) allele and the ADH2-10 promoter mutation should have no more activity than either single mutant. If Mot1 acts through a different location in the ADH2 promoter (directly at the TATA box, for example) or elsewhere in the repression pathway, then constitutive activity should be additive or greater in the ADH2-10 strain with a mutation in adr7-1. To test these alternatives, an adr7-1 ADH2-10 double mutant was constructed and ADH2 expression was assayed. ADHII activity in the double mutant is higher than in either single mutant alone (Table 4, compare lines 7, 6, and 2). The constitutive ADH2 expression in the ADH2-10 mutant and in the adr7-1ADH2-10 double mutant is SNF1 dependent, although the double mutant did exhibit a significantly higher level of SNF1-independent ADH2 expression than either single mutant (Table 4, compare lines 6 and 7 to lines 8 and 9). Deleting ADR1 in addition to SNF1 in the mot1 or mot1ADH2-10 mutants caused little further decrease in ADH2 expression, indicating that the SNF1-independent activity is also ADR1 independent (compare lines 10 and 11 to lines 8 and 9). Thus, ADH2-10 and MOT1 appear to affect ADH2 repression through different mechanisms, and the constitutive activity in both mutants requires Snf1.
Adr1 levels in constitutive mutants:
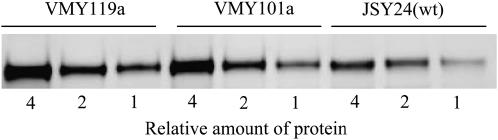
Since several of the VMY mutants are in vacuolar trafficking pathways and the vacuole is associated with protein degradation, we considered the possibility that the constitutive activity of ADH2 in the newly isolated mutant strains could be due to an increased steady state level of the Adr1 activator under repressed conditions. Very high levels of Adr1 can cause constitutive ADH2 expression (Denis 1987; Irani et al. 1987), and in the multicopy ADR1 strains used, Adr1 levels are elevated to derepressed levels. Although degradation of nonsecretory pathway proteins by the vacuole is unusual, it is not without precedent. A gluconeogenic enzyme that is required only under low-glucose conditions is degraded by import into vesicles for transport to the vacuole when glucose is abundant (Brown et al. 2002). To test this possibility, protein extracts of wild-type (JSY24) and VMY mutant strains were prepared and Western blots were performed. The amount of Adr1 under repressed conditions was determined by quantitative Western blotting for two or three mutants in each of the 12 complementation groups. Although some mutant strains showed two- to threefold elevated Adr1 levels, there were no consistent differences between mutant and wild-type strains (Figure 8 and supplemental Table 2 at http://www.genetics.org/supplemental/). We conclude that enhanced expression or stability of Adr1 is not responsible for the constitutive ADH2 expression in the mutants.
Figure 8.
Adr1 levels in ADH2 constitutive mutants. Extracts from mutant and wild-type (wt) strains were analyzed by Western blot as described in materials and methods. Two representatives from each complementation group were analyzed. Shown here are ADR14(VMY119a) and ADR16(VMY101a). Equal amounts of protein, in decreasing amounts of four-, two- and onefold, were loaded for each strain.
DISCUSSION
We identified and characterized a large collection of mutants that exhibit constitutive ADH2/lacZ expression in the presence of additional copies of the regulatory gene ADR1. In most of the mutants the endogenous ADH2 locus was also expressed constitutively and constitutive ADH2 expression was dependent on the major activator Adr1. Mutations in three complementation groups also partially alleviate SUC2 repression suggesting that these three genes have a general role in glucose repression.
Mutations in many genes not belonging to one of the 12 complementation groups also led to low-level resistance to glucose repression. One of these, mutant 13a, is allelic to ADR7, and another, 78a, is allelic to ADR8, both of which were identified in a previous genetic selection for glucose-insensitive ADH2 expression in the presence of an overexpressed, inactive ADR1-220 allele (Karnitz et al. 1992). Since only single representatives of these genes were identified, and many other genes are apparently represented only once in our collection, the screen is far from saturated. Thus, alteration of cellular physiology in numerous ways can lead to constitutive ADH2 expression when the requirement for the major activator Adr1 is satisfied. This may explain why ADR1 is normally expressed at very low levels under repressed growth conditions.
Cloning ADR7 (mutant 13a) revealed that it encodes the general repressor of polymerase II transcription, Mot1. MOT1 was identified in genetic screens for activation of transcription in the absence of an activator or a UAS sequence (Davis et al. 1992) and as an inhibitor of transcription in vitro (Auble and Hahn 1993). Mot1 can also act as an activator of basal transcription in vitro, and in vivo some genes are activated by the absence of MOT1 (Collart 1996). In vitro Mot1 acts to displace TBP from a TATA box in an ATP-dependent fashion (Auble et al. 1994). Mot1 interacts with the surface of TBP that interacts with DNA and thus acts as a competitive inhibitor of binding to a TATA box (Darst et al. 2001). In vivo Mot1 is thought to displace TBP from weak or non-productive sites and allow TBP to function more effectively from productive promoter sites (Muldrow et al. 1999).
The finding that ADR7 and MOT1 are allelic suggests that TBP binding to the ADH2 promoter may be inhibited by Mot1 during glucose repression. This idea is consistent with recent evidence indicating that Mot1 association with transcription components is regulated by environmental stress (Geisberg and Struhl 2004). After glucose depletion, the level or activity of Mot1 could be altered, resulting in expression of ADR1-dependent genes. Alternatively, the role of Mot1 in ADH2 repression may act through chromatin structure of the ADH2 promoter (Verdone et al. 1996) since Mot1 has a role in chromatin remodeling independent of its interaction with TBP (Topalidou et al. 2004). We failed to detect Mot1 at the ADH2 promoter using chromatin immunoprecipitation (C. Tachibana, unpublished data), suggesting that Mot1 may not act directly to repress TBP binding at the ADH2 promoter. For this reason we favor the possibility that Mot1 allows Snf1-dependent Adr1 binding and activation through an effect on chromatin structure, which is known to influence Adr1 binding (Verdone et al. 1996).
We cloned genes representing three other complementation groups not previously implicated in glucose repression. One of these, ADR22, is the FAB1 gene. FAB1 encodes PtdIns (3′)-P-(5′)-kinase (Yamamoto et al. 1995; Bonangelino et al. 2002; Gary et al. 2002). Fab1 is involved in cargo-selective sorting of membrane proteins to the lumen of the vacuole (Odorizzi et al. 2000), a process that requires its kinase activity. fab1 mutants have other defects as well, including a defect in nuclear morphology and osmotolerance (Bonangelino, et al. 2002) suggesting that it may have a role in cellular signaling. A second connection between ADH2 regulation and the vacuolar sorting pathway is the identification of VPS35 (ADR11) in our screen. VPS35 encodes a protein that is part of the retromer complex involved in sorting hydrolases to the vacuole (Paravicini et al. 1992; Seaman et al. 1998). The identification of mutations that alleviate glucose repression of ADH2 expression in a second gene in the sorting pathway suggests that vacuolar metabolism, rather than an unidentified function of Fab1, may cause relief from repression. However, impaired degradation of the major ADH2 activator, Adr1, is not responsible for constitutive ADH2 activation since the levels of Adr1 in wild-type vs. mutant whole-cell extracts are nearly equivalent.
An alternative possibility is that the effect of Fab1 on ADH2 expression is caused by an unknown cell-signaling function of Fab1. The substrate of the Fab1 kinase reaction, PtdIns (3′)-P, is the product of a PtdIns-3′-kinase encoded by VPS34 and deletion of VPS34 also causes constitutive ADH2 expression. This result suggests that the kinase activity of Fab1 is important for maintaining glucose repression, an interpretation consistent with the phenotype of a kinase-dead allele. Together, the results suggest that depleting the pool of phosphatidylinositol-3′,5′-phosphate relieves glucose repression. The pool of di- and triphosphoinositides in yeast is dramatically decreased when the energy charge of the cell is lowered, such as by glucose starvation (Lester and Steiner 1968). To date, however, these signaling molecules have not been associated in a causal manner with glucose repression.
Polyphosphoinositides have been implicated in gene expression through their effects on chromatin-remodeling activities (Odom et al. 2000; Saiardi et al. 2000; Steger et al. 2003). Since Adr1-dependent but transcription-independent chromatin remodeling accompanies ADH2 derepression (Verdone et al. 1997), if aberrant chromatin structure were induced in the fab1 mutant it could contribute to constitutive ADH2 expression.
The isolation of CDC10 and subsequent demonstration that two other genes involved in septin formation, SHS1 and ELM1, affect ADH2 regulation is the first suggestion that genes affecting 10-nm filaments may be involved in glucose signaling. Analysis of other septin mutants may reveal whether this is a direct and specific effect of these particular septin components or is an indirect consequence of the loss of septin assembly. Elm1 kinase is also an upstream kinase for Snf1, raising the possibility that the constitutive ADH2 expression in the ELM1 mutants acts through the SNF1 pathway of ADH2 regulation. Both loss-of-function and gain-of-function alleles of ELM1 cause constitutive ADH2 expression, suggesting that the activity of this pathway is very sensitive to the level of Elm1 activity since either too much or too little Elm1 causes a mutant phenotype. Mutations in two other Snf1 upstream kinases, Pak1 and Tos3, did not cause a constitutive ADH2 phenotype (N. Kacherovsky, unpublished data). Thus, it appears likely that the constitutive activity of ELM1 mutants acts through the septin pathway rather than through a SNF1-dependent activation pathway.
Our data provide the first evidence suggesting the involvement of the PtdIns (3′)-P-(5′)-kinase, Fab1, and septin assembly in glucose repression of the key gene involved in ethanol metabolism, ADH2. The possible genetic interactions between these genes, Mot1, Adr1, and Snf1 are shown in Figure 9A. An important question remaining is whether these genes act in a direct or an indirect manner to mediate repression of gene expression.
Figure 9.
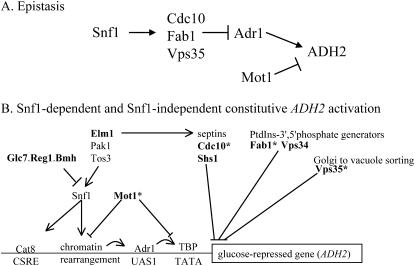
Interactions between factors affecting ADH2 expression. (A) Epistasis relationships between ADR1, SNF1, and CDC10, FAB1, VPS35, and MOT1 in ADH2 expression. Positive and negative interactions are shown by arrows and bars, respectively. (B) A model of ADH2 expression incorporating new regulatory genes. Boldface type indicates mutants are ADH2-constitutive. The asterisks indicate mutants isolated in this screen or in accompanying studies.
Together, these newly identified mutants outline at least two avenues from glucose depletion to transcriptional response that are dependent on Adr1 (Figure 9B). One involves the AMPkinase Snf1 and the repressor and chromatin remodeling enzyme Mot1. In the other, Snf1-independent route, the contributions of Cdc10, Fab1, and Vps35 to transcriptional regulation are less well-characterized, but may involve cell sensing and signaling of glucose and other stress conditions.
Acknowledgments
We thank J. Pringle and S. Emr for plasmids, D. Auble for tagged Mot1, M. Rose for plasmid libraries, K. Dombek for comments on the manuscript and discussion, and E. Arms for tetrad dissection. This research was supported by grant GM26079 from the National Institutes of Health.
References
- Auble, D. T., and S. Hahn, 1993. An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev. 7: 844–856. [DOI] [PubMed] [Google Scholar]
- Auble, D. T., K. E. Hansen, C. G. Mueller, W. S. Lane, J. Thorner et al., 1994. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 8: 1920–1934. [DOI] [PubMed] [Google Scholar]
- Auble, D. T., D. Wang, K. W. Post and S. Hahn, 1997. Molecular analysis of the SNF2/SWI2 protein family member MOT1, an ATP-driven enzyme that dissociates TATA-binding protein from DNA. Mol. Cell. Biol. 17: 4842–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg, H., A. Eisen, A. Sledziewski, D. Bader and E. T. Young, 1987. Two zinc fingers of a yeast regulatory protein shown by genetic evidence to be essential for its function. Nature 328: 443–445. [DOI] [PubMed] [Google Scholar]
- Boeke, J. D., J. Trueheart, G. Natsoulis and G. R. Fink, 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. [DOI] [PubMed] [Google Scholar]
- Bonangelino, C. J., J. J. Nau, J. E. Duex, M. Brinkman, A. E. Wurmser et al., 2002. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 156: 1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, C. R., J. A. McCann, G. G. Hung, C. P. Elco and H. L. Chiang, 2002. Vid22p, a novel plasma membrane protein, is required for the fructose-1,6-bisphosphatase degradation pathway. J. Cell Sci. 115: 655–666. [DOI] [PubMed] [Google Scholar]
- Burda, P., S. M. Padilla, S. Sarkar and S. D. Emr, 2002. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J. Cell Sci. 115: 3889–3900. [DOI] [PubMed] [Google Scholar]
- Carlson, M., 1999. Glucose repression in yeast. Curr. Opin. Microbiol. 2: 202–207. [DOI] [PubMed] [Google Scholar]
- Casamayor, A., and M. Snyder, 2003. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol. Cell. Biol. 23: 2762–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbon, G., K. D. Breunig, R. Wattiez, J. Vandenhaute and I. Noel-Georis, 2004. Key role of Ser562/661 in Snf1-dependent regulation of Cat8p in Saccharomyces cerevisiae and Kluyveromyces lactis. Mol. Cell. Biol. 24: 4083–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry, J. R., T. R. Johnson, C. Dollard, J. R. Shuster and C. L. Denis, 1989. Cyclic AMP-dependent protein kinase phosphorylates and inactivates the yeast transcriptional activator ADR1. Cell 56: 409–419. [DOI] [PubMed] [Google Scholar]
- Ciriacy, M., 1976. Cis-dominant regulatory mutations affecting the formation of glucose-repressible alcohol dehydrogenase (ADHII) in Saccharomyces cerevisiae. Mol. Gen. Genet. 145: 327–333. [DOI] [PubMed] [Google Scholar]
- Ciriacy, M., 1979. Isolation and characterization of further cis- and trans-acting regulatory elements involved in the synthesis of glucose-repressible alcohol dehydrogenase (ADHII) in Saccharomyces cerevisiae. Mol. Gen. Genet. 176: 427–431. [DOI] [PubMed] [Google Scholar]
- Collart, M. A., 1996. The NOT, SPT3, and MOT1 genes functionally interact to regulate transcription at core promoters. Mol. Cell. Biol. 16: 6668–6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst, R. P., D. Wang and D. T. Auble, 2001. MOT1-catalyzed TBP-DNA disruption: uncoupling DNA conformational change and role of upstream DNA. EMBO J. 20: 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. L., R. Kunisawa and J. Thorner, 1992. A presumptive helicase (MOT1 gene product) affects gene expression and is required for viability in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 12: 1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, C. L., 1984. Identification of new genes involved in the regulation of yeast alcohol dehydrogenase II. Genetics 108: 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, C. L., 1987. The effects of ADR1 and CCR1 gene dosage on the regulation of the glucose-repressible alcohol dehydrogenase from Saccharomyces cerevisiae. Mol. Gen. Genet. 208: 101–106. [DOI] [PubMed] [Google Scholar]
- Denis, C. L., and C. Gallo, 1986. Constitutive RNA synthesis for the yeast activator ADR1 and identification of the ADR1–5c mutation: implications in posttranslational control of ADR1. Mol. Cell. Biol. 6: 4026–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRisi, J. L., V. R. Iyer and P. O. Brown, 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278: 680–686. [DOI] [PubMed] [Google Scholar]
- Dombek, K. M., and E. T. Young, 1997. Cyclic AMP-dependent protein kinase inhibits ADH2 expression in part by decreasing expression of the transcription factor gene ADR1. Mol. Cell. Biol. 17: 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek, K. M., S. Camier and E. T. Young, 1993. ADH2 expression is repressed by REG1 independently of mutations that alter the phosphorylation of the yeast transcription factor ADR1. Mol. Cell. Biol. 13: 4391–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek, K. M., V. Voronkova, A. Raney and E. T. Young, 1999. Functional analysis of the yeast Glc7-binding protein Reg1 identifies a protein phosphatase type 1-binding motif as essential for repression of ADH2 expression. Mol. Cell. Biol. 19: 6029–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek, K. M., N. Kacherovsky and E. T. Young, 2004. The Reg1-interacting proteins, Bmh1, Bmh2, Ssb1, and Ssb2, have roles in maintaining glucose repression in Saccharomyces cerevisiae. J. Biol. Chem. 279: 39165–39174. [DOI] [PubMed] [Google Scholar]
- Flescher, E., K. Madden and M. Snyder, 1993. Componens required for cytokinesis are important for bud site selection in yeast. J. Cell Biol. 122: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, J. A., M. L. Wong, M. S. Longtine, J. R. Pringle, M. Mann et al., 1998. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 143: 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo, J. M., 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62: 334–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary, J. D., T. K. Sato, C. J. Stefan, C. J. Bonangelino, L. S. Weisman et al., 2002. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol. Biol. Cell 13: 1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisberg, J. V., and K. Struhl, 2004. Cellular stress alters the transcriptional properties of promoter-bound Mot1-TBP complexes. Mol. Cell 14: 479–489. [DOI] [PubMed] [Google Scholar]
- Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles et al., 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. [DOI] [PubMed] [Google Scholar]
- Guarente, L., 1983. Yeast Promoters and lacZ Fusions Designed to Study Expression of Cloned Genes in Yeast. (Methods in Enzymology, Vol. 101, pp. 181–191). Academic Press, San Diego. [DOI] [PubMed]
- Guldener, U., S. Heck, T. Fiedler, J. Beinhauer and J. H. Hegemann, 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24: 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and J. R. Fink, 2002. Guide to Yeast Genetics and Molecular Biology. (Methods in Enzymology, Vol. 350). Academic Press, San Diego.
- Hardie, D. G., D. Carling and M. Carlson, 1998. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67: 821–855. [DOI] [PubMed] [Google Scholar]
- Hartwell, L., 1971. Genetic control of the cell division cycle in yeast IV.Genes controlling bud emergence and cytokinesis. Exp. Cell Res.. 69: 265–276. [DOI] [PubMed] [Google Scholar]
- Hong, S. P., F. C. Leiper, A. Woods, D. Carling and M. Carlson, 2003. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. USA 100: 8839–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani, M., W. E. Taylor and E. T. Young, 1987. Transcription of the ADH2 gene in Saccharomyces cerevisiae is limited by positive factors that bind competitively to its intact promoter region on multicopy plasmids. Mol. Cell. Biol. 7: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, R., and M. Carlson, 1997. The Snf1 protein kinase and its activating subunit, Snf4, interact with distinct domains of the Sip1/Sip2/Gal83 component in the kinase complex. Mol. Cell. Biol. 17: 2099–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnitz, L., M. Morrison and E. T. Young, 1992. Identification and characterization of three genes that affect expression of ADH2 in Saccharomyces cerevisiae. Genetics 132: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnirov, V., 2000. Rapid and reliable protein extraction from yeast. Yeast 16: 857–860. [DOI] [PubMed] [Google Scholar]
- Lester, R., and M. R. Steiner, 1968. The occurrence of diphosphoinositides and triphosphoinositides in Saccharomyces cerevisiae. J. Biol. Chem. 243: 4889–4893. [PubMed] [Google Scholar]
- Longtine, M. S., and E. Bi, 2003. Regulation of septin organization and function in yeast. Trends Cell Biol. 13: 403–409. [DOI] [PubMed] [Google Scholar]
- Muldrow, T. A., A. M. Campbell, P. A. Weil and D. T. Auble, 1999. MOT1 can activate basal transcription in vitro by regulating the distribution of TATA binding protein between promoter and nonpromoter sites. Mol. Cell. Biol. 19: 2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom, A. R., A. Stahlberg, S. R. Wente and J. D. York, 2000. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 287: 2026–2029. [DOI] [PubMed] [Google Scholar]
- Odorizzi, G., M. Babst and S. D. Emr, 2000. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem. Sci. 25: 229–235. [DOI] [PubMed] [Google Scholar]
- Paravicini, G., B. F. Horazdovsky and S. D. Emr, 1992. Alternative pathways for the sorting of soluble vacuolar proteins in yeast: a vps35 null mutant missorts and secretes only a subset of vacuolar hydrolases. Mol. Biol. Cell 3: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner, A., A. Scholer, E. Martens, B. Gollwitzer and H. J. Schuller, 1996. Dual influence of the yeast Cat1p (Snf1p) protein kinase on carbon source-dependent transcriptional activation of gluconeogenic genes by the regulatory gene CAT8. Nucleic Acids Res. 24: 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner, A., M. Hiesinger and H. J. Schuller, 1999. Deregulation of gluconeogenic structural genes by variants of the transcriptional activator Cat8p of the yeast Saccharomyces cerevisiae. Mol. Microbiol. 34: 146–156. [DOI] [PubMed] [Google Scholar]
- Russell, D. W., M. Smith, D. Cox, V. M. Williamson and E. T. Young, 1983. DNA sequences of two yeast promoter-up mutants. Nature 304: 652–654. [DOI] [PubMed] [Google Scholar]
- Saiardi, A., J. J. Caffrey, S. H. Snyder and S. B. Shears, 2000. The inositol hexakisphosphate kinase family. Catalytic flexibility and function in yeast vacuole biogenesis. J. Biol. Chem. 275: 24686–24692. [DOI] [PubMed] [Google Scholar]
- Sanz, P., G. R. Alms, T. A. Haystead and M. Carlson, 2000. Regulatory interactions between the Reg1-Glc7 protein phosphatase and the Snf1 protein kinase. Mol. Cell. Biol. 20: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M. C., and R. R. McCartney, 2000. beta-subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J. 19: 4936–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller, H. J., 2003. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr. Genet. 43: 139–160. [DOI] [PubMed] [Google Scholar]
- Seaman, M. N., J. M. McCaffery and S. D. Emr, 1998. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142: 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan, J. S., K. M. Dombek and E. T. Young, 1999. Post-translational regulation of Adr1 activity is mediated by its DNA binding domain. J. Biol. Chem. 274: 37575–37582. [DOI] [PubMed] [Google Scholar]
- Sreenivasan, A., and D. Kellogg, 1999. The elm1 kinase functions in a mitotic signaling network in budding yeast. Mol. Cell. Biol. 19: 7983–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger, D. J., E. S. Haswell, A. L. Miller, S. R. Wente and E. K. O'Shea, 2003. Regulation of chromatin remodeling by inositol polyphosphates. Science 299: 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana, C., J. Y. Yoo, J. B. Tagne, N. Kacherovsky, T. I. Lee et al., 2005. Combined global localization analysis and transcriptome data identify genes that are directly coregulated by Adr1 and Cat8. Mol. Cell. Biol. 25: 2138–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalidou, I., M. Papamichos-Chronakis, G. Thireos and D. Tzamarias, 2004. Spt3 and Mot1 cooperate in nucleosome remodeling independently of TBP recruitment. EMBO J. 23: 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone, L., G. Camilloni, E. Di Mauro and M. Caserta, 1996. Chromatin remodeling during Saccharomyces cerevisiae ADH2 gene activation. Mol. Cell. Biol. 16: 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone, L., F. Cesari, C. L. Denis, E. Di Mauro and M. Caserta, 1997. Factors affecting Saccharomyces cerevisiae ADH2 chromatin remodeling and transcription. J. Biol. Chem. 272: 30828–30834. [DOI] [PubMed] [Google Scholar]
- Verdone, L., J. Wu, K. van Riper, N. Kacherovsky, M. Vogelauer et al., 2002. Hyperacetylation of chromatin at the ADH2 promoter allows Adr1 to bind in repressed conditions. EMBO J. 21: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, K., and H. J. Schuller, 2001. Adr1 and Cat8 synergistically activate the glucose-regulated alcohol dehydrogenase gene ADH2 of the yeast Saccharomyces cerevisiae. Microbiology 147: 2037–2044. [DOI] [PubMed] [Google Scholar]
- Williamson, V. M., J. Bennetzen, E. T. Young, K. Nasmyth and B. D. Hall, 1980. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature 283: 214–216. [DOI] [PubMed] [Google Scholar]
- Williamson, V. M., E. T. Young and M. Ciriacy, 1981. Transposable elements associated with constitutive expression of yeast alcohol dehydrogenase II. Cell 23: 605–614. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A., D. B. DeWald, I. V. Boronenkov, R. A. Anderson, S. D. Emr et al., 1995. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell 6: 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, E. T., N. Kacherovsky and K. Van Riper, 2002. Snf1 Protein Kinase Regulates Adr1 Binding to Chromatin but Not Transcriptional Activation. J. Biol. Chem. 277: 38095–38103. [DOI] [PubMed] [Google Scholar]
- Young, E. T., K. M. Dombek, C. Tachibana and T. Ideker, 2003. Multiple pathways are co-regulated by the protein kinase Snf1 and the transcription factors Adr1 and Cat8. J. Biol. Chem. 278: 26146–26158. [DOI] [PubMed] [Google Scholar]