Abstract
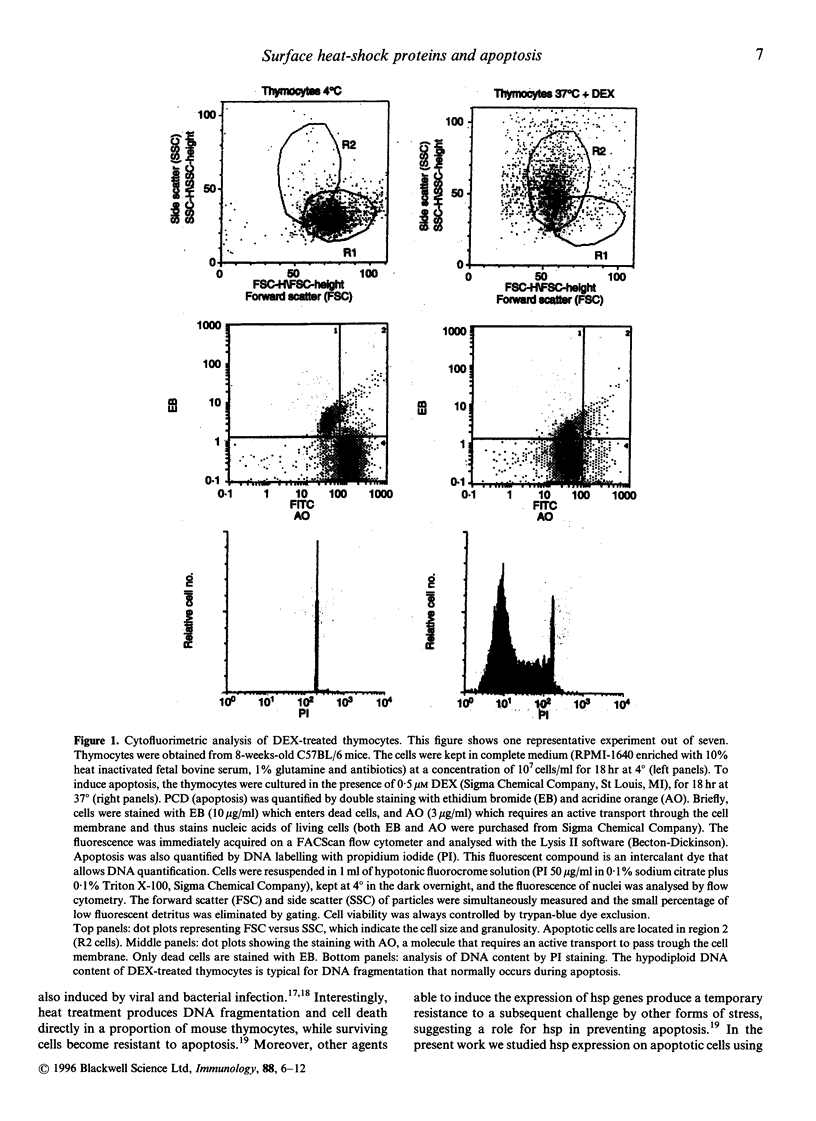
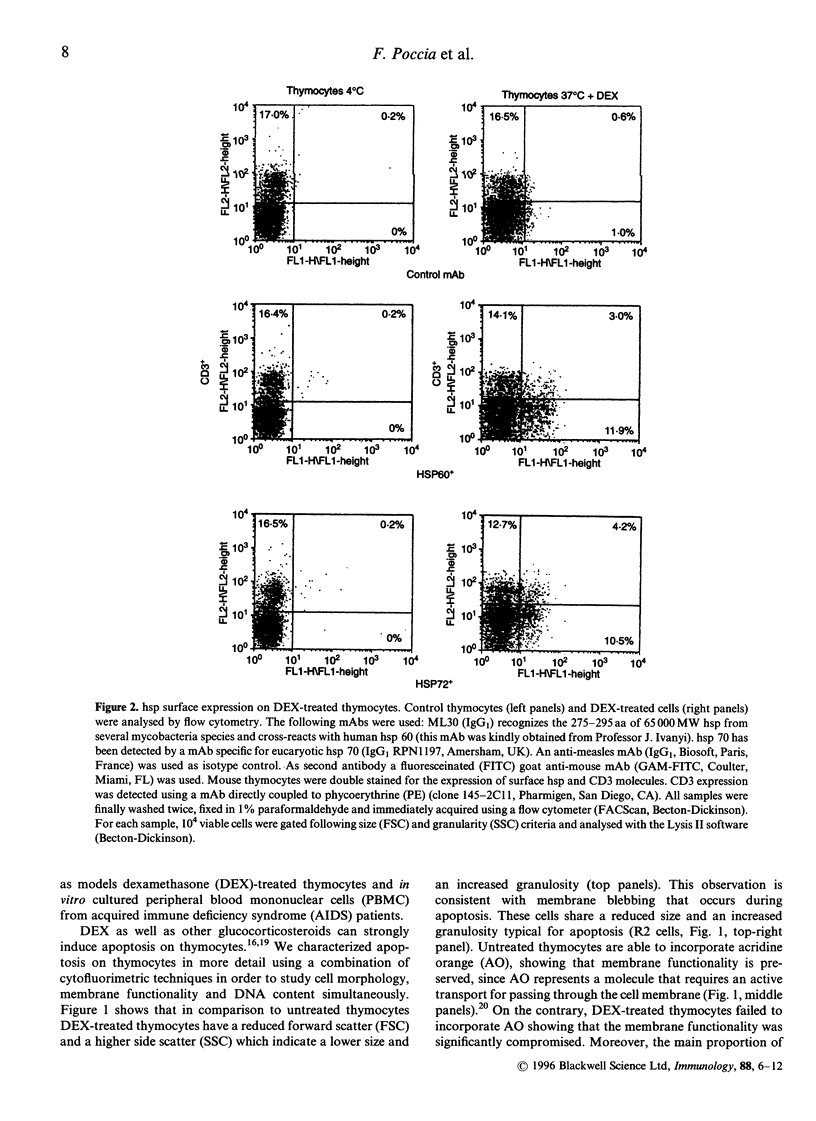
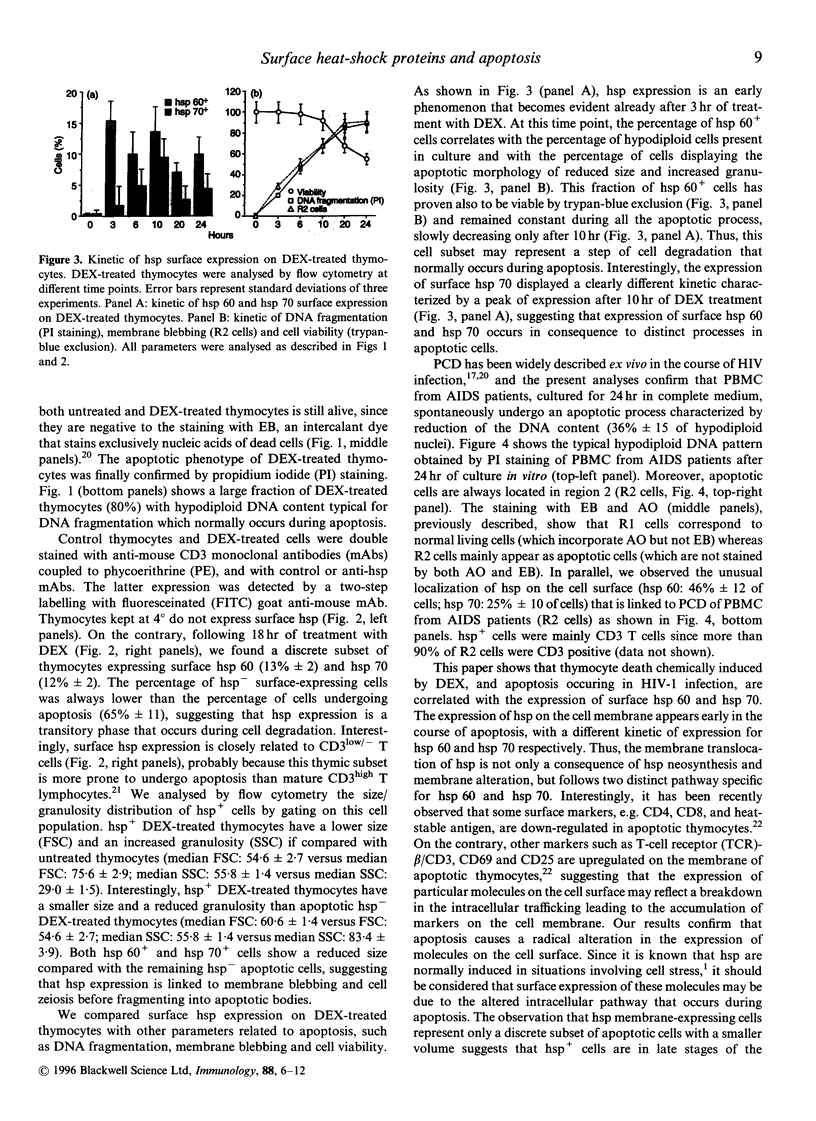
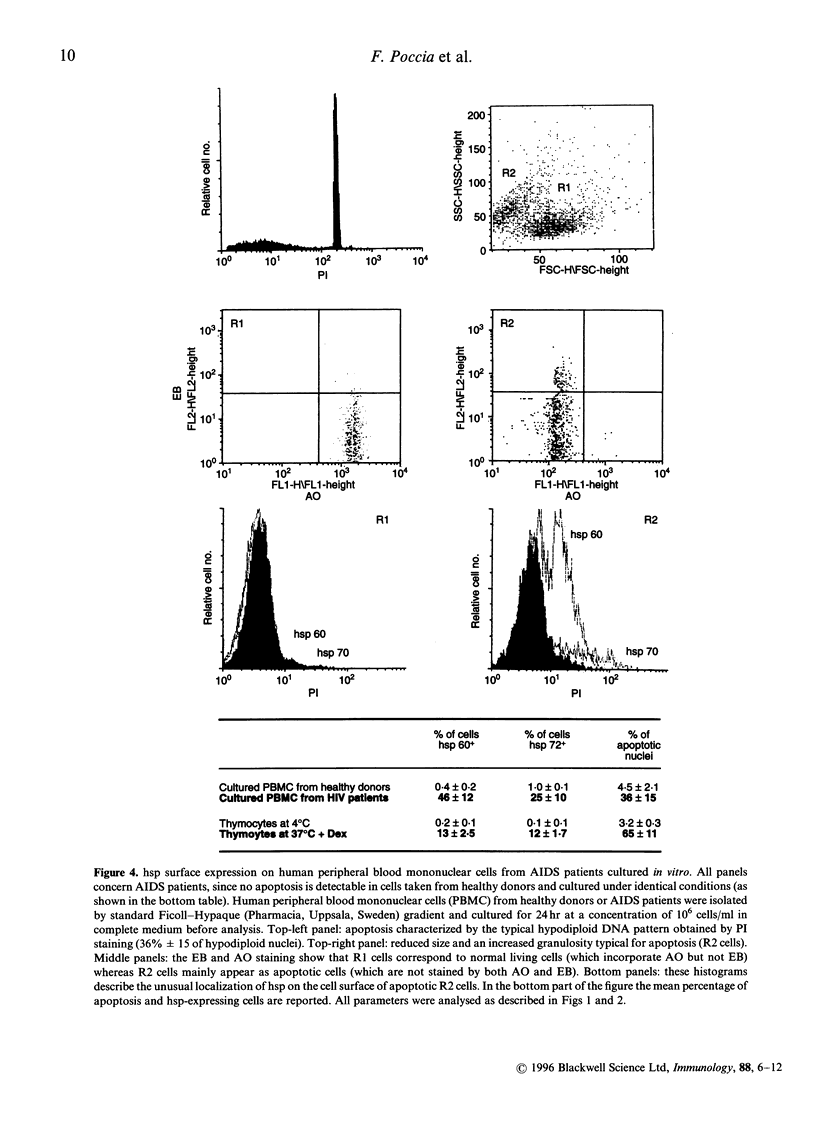
Heat-shock proteins (hsp) represent a highly conserved family of proteins, normally localized in the cytoplasm and nucleus, whose expression is induced in situations involving cell stress. This paper reports the unusual translocation of hsp to the cell membrane of T cells undergoing apoptosis. We observed that glucocorticosteroid-induced thymocyte death is associated to the surface expression of hsp 60 and hsp 70 in a discrete fraction of apoptotic cells. hsp surface expression is closely related to a thymic subset of immature CD3low/- T cells. The expression of surface hsp 60 appears early after treatment with dexamethasone (3 hr) whereas the membrane expression of hsp 70 follows different kinetics and peaks later. Morphological analysis of the hsp+ apoptotic cells suggest that this subset represents late-stage apoptotic cells at their minimal volume before fragmentation into apoptotic bodies. Membrane expression of hsp is also associated with apoptosis in peripheral blood mononuclear cells from AIDS patients cultured in vitro. Altogether, we show that a discrete fraction of cells undergoing apoptosis expresses membrane hsp 60 and hsp 70, supporting the hypothesis that apoptosis causes a radical alteration in the expression of cell surface molecules. Surface hsp expressed during apoptosis may constitute a novel immune-context able to generate packages of self- and exogenous antigens, originating from degradation of altered cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung R. K., Dosch H. M. The growth transformation of human B cells involves superinduction of hsp70 and hsp90. Virology. 1993 Apr;193(2):700–708. doi: 10.1006/viro.1993.1178. [DOI] [PubMed] [Google Scholar]
- Chouchane L., Bowers F. S., Sawasdikosol S., Simpson R. M., Kindt T. J. Heat-shock proteins expressed on the surface of human T cell leukemia virus type I-infected cell lines induce autoantibodies in rabbits. J Infect Dis. 1994 Feb;169(2):253–259. doi: 10.1093/infdis/169.2.253. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNagel D. C., Pierce S. K. A case for chaperones in antigen processing. Immunol Today. 1992 Mar;13(3):86–89. doi: 10.1016/0167-5699(92)90147-Y. [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Heltai S., Zocchi M. R., Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992 Jun 19;51(4):613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Furlini G., Vignoli M., Re M. C., Gibellini D., Ramazzotti E., Zauli G., La Placa M. Human immunodeficiency virus type 1 interaction with the membrane of CD4+ cells induces the synthesis and nuclear translocation of 70K heat shock protein. J Gen Virol. 1994 Jan;75(Pt 1):193–199. doi: 10.1099/0022-1317-75-1-193. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Ulug E. T., Bose H. R., Jr Induction of stress proteins in Sindbis virus- and vesicular stomatitis virus-infected cells. Virology. 1983 Sep;129(2):319–332. doi: 10.1016/0042-6822(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Hainaut P., Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992 Oct;11(10):3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Weinberg R. A. An interaction between p21ras and heat shock protein hsp60, a chaperonin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2012–2016. doi: 10.1073/pnas.89.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur I., Voss S. D., Gupta R. S., Schell K., Fisch P., Sondel P. M. Human peripheral gamma delta T cells recognize hsp60 molecules on Daudi Burkitt's lymphoma cells. J Immunol. 1993 Mar 1;150(5):2046–2055. [PubMed] [Google Scholar]
- Kishimoto H., Surh C. D., Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995 Feb 1;181(2):649–655. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaThangue N. B., Shriver K., Dawson C., Chan W. L. Herpes simplex virus infection causes the accumulation of a heat-shock protein. EMBO J. 1984 Feb;3(2):267–277. doi: 10.1002/j.1460-2075.1984.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L. S., Hsu S., Vaewhongs M., Wu B. The hsp70 gene CCAAT-binding factor mediates transcriptional activation by the adenovirus E1a protein. Mol Cell Biol. 1992 Jun;12(6):2599–2605. doi: 10.1128/mcb.12.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara G. C., Sansoni P., Badiali-De Giorgi L., Gallinella G., Ferrari C., Brianti V., Fagnoni F. F., Ruegg C. L., De Panfilis G., Pasquinelli G. New insights suggesting a possible role of a heat shock protein 70-kD family-related protein in antigen processing/presentation phenomenon in humans. Blood. 1993 Nov 1;82(9):2865–2871. [PubMed] [Google Scholar]
- Migliorati G., Nicoletti I., Crocicchio F., Pagliacci C., D'Adamio F., Riccardi C. Heat shock induces apoptosis in mouse thymocytes and protects them from glucocorticoid-induced cell death. Cell Immunol. 1992 Sep;143(2):348–356. doi: 10.1016/0008-8749(92)90031-j. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy A., Laochumroonvorapong P., Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994 Oct 1;180(4):1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G., Botzler C., Wiesnet M., Müller E., Meier T., Wilmanns W., Issels R. D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995 Apr 10;61(2):272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Muro-Cacho C. A., Pantaleo G., Fauci A. S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995 May 15;154(10):5555–5566. [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Phillips B., Abravaya K., Morimoto R. I. Analysis of the specificity and mechanism of transcriptional activation of the human hsp70 gene during infection by DNA viruses. J Virol. 1991 Nov;65(11):5680–5692. doi: 10.1128/jvi.65.11.5680-5692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini M. Tissue transglutaminase: a candidate effector element of physiological cell death. Curr Top Microbiol Immunol. 1995;200:163–175. doi: 10.1007/978-3-642-79437-7_12. [DOI] [PubMed] [Google Scholar]
- Poccia F., Piselli P., Di Cesare S., Bach S., Colizzi V., Mattei M., Bolognesi A., Stirpe F. Recognition and killing of tumour cells expressing heat shock protein 65 kD with immunotoxins containing saporin. Br J Cancer. 1992 Sep;66(3):427–432. doi: 10.1038/bjc.1992.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Casciola-Rosen L., Ahearn J. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995 Apr 1;181(4):1557–1561. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y., Tsuboi N., Sato N., Kikuchi K. 70 kDa heat shock cognate protein is a transformation-associated antigen and a possible target for the host's anti-tumor immunity. J Immunol. 1993 Nov 15;151(10):5516–5524. [PubMed] [Google Scholar]
- Tanaka Y., Morita C. T., Tanaka Y., Nieves E., Brenner M. B., Bloom B. R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Tiso M., Gangemi R., Bargellesi Severi A., Pizzolitto S., Fabbi M., Risso A. Spontaneous apoptosis in human thymocytes. Am J Pathol. 1995 Aug;147(2):434–444. [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]