Abstract
Drosophila yakuba is widely distributed in sub-Saharan Africa, while D. santomea is endemic to the volcanic island of São Tomé in the Atlantic Ocean, 280 km west of Gabon. On São Tomé, D. yakuba is found mainly in open lowland forests, and D. santomea is restricted to the wet misty forests at higher elevations. At intermediate elevations, the species form a hybrid zone where hybrids occur at a frequency of ∼1%. To determine the extent of gene flow between these species we studied polymorphism and divergence patterns in 29 regions distributed throughout the genome, including mtDNA and three genes on the Y chromosome. This multilocus approach, together with the comparison to the two allopatric species D. mauritiana and D. sechellia, allowed us to distinguish between forces that should affect all genes and forces that should act on some genes (e.g., introgression). Our results show that D. yakuba mtDNA has replaced that of D. santomea and that there is also significant introgression for two nuclear genes, yellow and salr. The majority of genes, however, has remained distinct. These two species therefore do not form a “hybrid swarm” in which much of the genome shows substantial introgression while disruptive selection maintains distinctness for only a few traits (e.g., pigmentation and male genitalia).
ACCORDING to the biological species concept (BSC), the coexistence of distinct entities in sympatry suggests a severe reduction in gene flow between them (Dobzhansky 1937; Mayr 1942; Coyne 1992; Coyne and Orr 1998). It is clear, however, that there is more introgression between species than early advocates of the BSC suspected (Coyne and Orr 2004). This observation of introgression has prompted the proposal of alternative species concepts. Mallet (1995), for example, proposed the “genotypic cluster species concept” (GCSC), in which factors beyond reproductive isolation, such as stabilizing selection or historical inertia, are claimed to maintain discrete species in one habitat. As Coyne and Orr (2004) noted, many of these “other factors” are in fact forms of reproductive isolation. The main difference between the BSC and many proposed alternatives—including the GCSC and Wu's (2001) “genic species concept”—is in how much gene flow between sympatric entities can occur while those entities still remain distinct. Sympatric, recognizably distinct clusters, for example, may differ at only a few loci while exchanging genes freely throughout the rest of the genome.
For several reasons it is important to determine the degree of gene exchange between sympatric or parapatric clusters. First, the evolutionary independence of such taxa depends on the degree to which they can exchange generally adaptive alleles. Second, we want to know whether specific regions of the genome introgress more readily than others. For example, regions linked to genes causing species-specific adaptations or hybrid sterility may be limited in their ability to move between species (Tucker et al. 1992; Machado et al. 2002). Chromosomal rearrangements that differ between species may also serve as “traps” for genes causing hybrid incompatibilities and thus also show limited introgression (Rieseberg et al. 1995; Noor et al. 2001; Machado et al. 2002; Navarro and Barton 2003). Third, it has been claimed that introgression can be a source of genetic variation that allows species to adapt to new environments and hence can serve as an engine of adaptation (Arnold 1997). Finally, we want to determine on the genic level the applicability of various species concepts: for example, Are the genomes of sympatric, hybridizing species largely impermeable to new variation, as the BSC might predict?
Answers to these questions have generally come from hybrid zones, which show in general that introgression, while more frequent than previously suspected, is limited between sympatric taxa (Coyne and Orr 2004). Unfortunately, there is a dearth of hybrid zones in the most genetically well-studied group, Drosophila. Lachaise et al. (2000), however, recently described a hybrid zone between the sister species Drosophila yakuba and D. santomea on the Island of São Tomé, a small volcanic island 280 km off the coast of Gabon. D. yakuba is widely distributed in sub-Saharan Africa and in the islands near the continent (including Madagascar), while D. santomea is endemic to São Tomé. The most striking morphological difference between D. yakuba and D. santomea is the pigmentation pattern: D. yakuba has the characteristic pattern of the D. melanogaster group (females' yellow abdomens are striped with black, while those of males have black tips), while D. santomea completely lacks this dark pigmentation (Lachaise et al. 2000; Llopart et al. 2002). Other traits, such as male genital morphology and the number of sex comb teeth, also distinguish these species (Lachaise et al. 2000; Coyne et al. 2004). Moreover, D. yakuba is highly polymorphic for chromosome inversions, with the right arm of chromosome 2 showing the most polymorphism (Lemeunier and Ashburner 1976). In contrast, D. santomea shows no polymorphism for chromosomal arrangements, and no fixed inversions distinguish D. yakuba and D. santomea (Lachaise et al. 2000). Molecular data suggest that D. santomea arose on the island after the colonization by its common ancestor with D. yakuba ∼400,000 years ago (Cariou et al. 2001; Llopart et al. 2002). Tentative molecular evidence suggests that the current presence of D. yakuba on the island reflects a more recent colonization (Cariou et al. 2001).
As expected from its status as an open forest/savannah species, D. yakuba usually occurs at low elevations on São Tomé, mainly in areas cleared by humans beginning in the 16th century. In contrast, D. santomea inhabits the wet forests found at higher elevations. On the largest mountain in the island, Pico de São Tomé, D. yakuba is found only below 1450 m, while D. santomea occurs between 1150 and 2024 m. Between 1150 and 1450 m—an area that coincides with the transition between agricultural land and virgin rainforest—the species coexist, with the abundance ratio of D. yakuba/D. santomea changing from 2 to 0.05 as one moves upward through the zone (Lachaise et al. 2000). Hybrids have been reported in this zone at a frequency of ∼1%; these were diagnosed as hybrids by their possession of intermediate genitalia and pigmentation. In the laboratory, these species show several forms of reproductive isolation, which include mate discrimination (Lachaise et al. 2000; Coyne et al. 2002), conspecific sperm precedence (Chang 2004), and sterility that conforms to Haldane's rule: F1 hybrid males are completely sterile and F1 hybrid females are partly or fully fertile (Coyne et al. 2004).
Here, we report the patterns of polymorphism and divergence between D. yakuba and D. santomea on the basis of the examination of a substantial number of genes spread throughout the genome. Our aim is to determine the extent of introgression for nuclear and mitochondrial genomes and to compare the amount and patterns of introgression with those predicted from laboratory data on the genetics of hybrid sterility. Because our multilocus approach investigates genome-wide variation, it allows us to distinguish between forces that should affect all genes and forces that should act on some but not all genes (e.g., introgression). This multilocus approach, pioneered by J. Hey and colleagues, has proved useful in revealing the pattern and extent of gene exchange in nature (Hey and Kliman 1993; Hilton et al. 1994; Wang et al. 1997; Kliman et al. 2000; Machado et al. 2002; Broughton and Harrison 2003; Ramos-Onsins et al. 2004).
MATERIALS AND METHODS
Loci and flies analyzed:
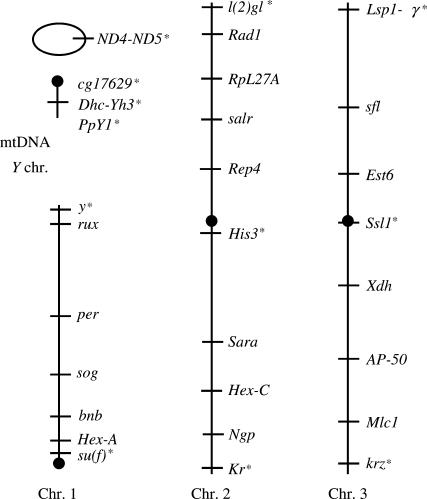
We used D. yakuba or D. melanogaster sequences to design primers to sequence 45 nuclear genes (Table 1) in D. yakuba Taï18 and D. santomea STO.4 strains (Llopart et al. 2002). Polymorphism data in both species were collected for 28 out of 45 randomly selected nuclear genes and the mitochondrial region ND5-ND4 (see Figure 1), with a sample size ranging from 21 to 32 chromosomes depending on the loci (Table 2). The “sympatric” specimens of D. yakuba and D. santomea were captured in the hybrid zone (elevation 1250 m) in the Obo Natural Reserve in March 2003 on São Tomé Island. At the same time, “allopatric” individuals of D. yakuba and D. santomea were collected, respectively, in a garden outside São Tomé City (elevation 5 m) and on Pico Calvario (a shoulder on the main mountain of Pico de São Tomé at 1566 m). In each of these locations only one species is found, although on Pico Calvario D. yakuba occurs within a few kilometers. Upon collection, all flies were immediately preserved in absolute ethanol until DNA extraction was performed in the laboratory. For mtDNA and Y chromosome genes, we analyzed a more geographically diverse sample of isofemale lines. The D. yakuba lines include strains from the Ivory Coast (Taï18 and TM34), Cameroon (CAM), Gabon (GAB), and Principé Island (ANTON-1) and from the zones of allopatry (SJ1 and BAR2) and sympatry (BOSU, COST2, SA1, and OBAT5) with D. santomea on São Tomé Island. For D. santomea, the sample includes one allopatric isofemale line (CAR1566.6) from Pico Calvario (elevation 1566 m) and nine additional sympatric lines. Eight of these were collected in the hybrid zone on Pico de São Tomé (STO.4, STO.10, STO.15, STO.18, COST 1235.1, OBAT 1200.13, OBAT 1200.14, and LAGO 1482.11), and one (QUIJA 650.1) came from Rio Quija, in southwest São Tomé, an area that also harbors D. yakuba. More detailed information on some of these strains can be found in Coyne et al. (2002). We also used the isofemale strains D. mauritiana B (from Mauritius Island) and D. sechellia SY 001 (from the Seychelles archipelago) to distinguish between selective constraints and introgression as explanations for sequence similarity in regions of low frequency of crossing over. To assess introgression between D. yakuba and D. teissieri, we also sequenced the ND5-ND4 mitochondrial region and the Y chromosome genes in the Brazzaville 8 strain (collected in the Republic of the Congo).
TABLE 1.
Synonymous (Ks) and nonsynonymous (Ka) substitutions per site between D. yakuba and D. santomea
| Gene | Ks | Ka | Size (bp)b |
|---|---|---|---|
| bnba | 0.047 | 0.0033 | 1248 |
| f | 0.063 | 0.0047 | 843 |
| Hex-Aa | 0.018 | 0 | 1290 |
| pera | 0.097 | 0 | 801 |
| ruxa | 0.066 | 0.027 | 945 |
| sn | 0.04 | 0 | 1134 |
| soga | 0.033 | 0 | 1200 |
| su(f)a | 0.021 | 0 | 414 |
| ya | 0.0029 | 0 | 1041 |
| CG17629a | 0.023 | 0 | 936 |
| Dhc-Yh3a | 0.0104 | 0 | 924 |
| Pp1Y1a | 0.017 | 0 | 486 |
| Adh | 0.0047 | 0.004 | 630 |
| barr | 0.033 | 0.0057 | 954 |
| Hex-Ca | 0.14 | 0.0022 | 1272 |
| His3a | 0.0073 | 0 | 408 |
| Kra | 0.013 | 0.0056 | 1350 |
| l(2)gla | 0.033 | 0.0027 | 903 |
| Ngpa | 0.029 | 0.0037 | 741 |
| Pgi | 0.013 | 0 | 717 |
| Rad1a | 0.054 | 0.0016 | 741 |
| Rep4a | 0.063 | 0.0053 | 846 |
| RpL27Aa | 0.015 | 0.0029 | 414 |
| salra | 0.045 | 0.0012 | 1005 |
| Saraa | 0.036 | 0.0073 | 915 |
| trp1 | 0.062 | 0.0027 | 1011 |
| vkg | 0.023 | 0.014 | 867 |
| AP-50a | 0.039 | 0 | 1128 |
| dib | 0.097 | 0.0027 | 1359 |
| dos | 0.032 | 0 | 867 |
| Est6a | 0.077 | 0.011 | 600 |
| hb | 0.036 | 0.0005 | 2199 |
| Hsc70-4 | 0.018 | 0 | 1086 |
| Lsp1-γa | 0.027 | 0 | 597 |
| Mlc1a | 0.091 | 0 | 78 |
| Pgm | 0.053 | 0.0012 | 1185 |
| RpL14 | 0.019 | 0 | 411 |
| Rpn5 | 0.039 | 0.0012 | 963 |
| sfla | 0.09 | 0 | 864 |
| Sod | 0.13 | 0.0034 | 360 |
| Ssl1a | 0.033 | 0.0037 | 654 |
| Xdha | 0.051 | 0.0031 | 1269 |
| ymp | 0.066 | 0.0078 | 504 |
| Totalc | 0.044 | 0.0029 | 38160 |
Loci included in the polymorphism survey.
Size of the coding region analyzed.
Divergence based on the concatenated sequence including the regions AnnX and v, 12 and 18 bp long, respectively.
Figure 1.
Chromosomal locations of the loci included in the polymorphism survey. Cytological positions were inferred from D. melanogaster and the comparative salivary-gland banding maps of Lemeunier and Ashburner (1976). Solid circles symbolize the centromeric regions of the chromosomes. Loci in regions of reduced crossing over are indicated by asterisks.
TABLE 2.
Polymorphism data summary
| Locus | Species | na | Sb | Sync | Repc | θtd | θsd | De | L (bp)f |
|---|---|---|---|---|---|---|---|---|---|
| A. Regions of reduced frequency of crossing over | |||||||||
| ND5–ND4 | D. yakuba | 11 | 8 | 6 | 2 | 0.0016 | 0.0046 | −1.27 | 1657 |
| D. santomea | 10 | 4 | 4 | 0 | 0.00085 | 0.0032 | −0.52 | ||
| Dhc-Yh3 | D. yakuba | 11 | 0 | 0 | 0 | 0 | 0 | — | 1219 |
| D. santomea | 10 | 0 | 0 | 0 | 0 | 0 | — | ||
| CG17629 | D. yakuba | 11 | 1 | 1 | 0 | 0.0004 | 0.0016 | 0.67 | 936 |
| D. santomea | 10 | 3 | 1 | 2 | 0.0011 | 0.001 | −1.03 | ||
| Pp1Y1 | D. yakuba | 11 | 1 | 0 | 1 | 0.0007 | 0 | −1.13 | 490 |
| D. santomea | 10 | 0 | 0 | 0 | 0 | 0 | — | ||
| y | D. yakuba | 16 | 6 | 6 | 0 | 0.0019 | 0.0078 | −1.17 | 965 |
| D. santomea | 16 | 0 | 0 | 0 | 0 | 0 | — | ||
| su(f) | D. yakuba | 16 | 1 | 0 | 0 | 0.0005 | 0.0008 | 0.15 | 607 |
| D. santomea | 16 | 0 | 0 | 0 | 0 | 0 | — | ||
| l(2)gl | D. yakuba | 16 | 1 | 1 | 0 | 0.0005 | 0.0021 | 1.03 | 608 |
| D. santomea | 16 | 3 | 2 | 1 | 0.0015 | 0.0042 | 0.17 | ||
| His3 | D. yakuba | 16 | 3 | 0 | 0 | 0.0018 | 0.0022 | −0.65 | 496 |
| D. santomea | 16 | 0 | 0 | 0 | 0 | 0 | — | ||
| Kr | D. yakuba | 16 | 3 | 3 | 0 | 0.002 | 0.0086 | 0.01 | 456 |
| D. santomea | 16 | 0 | 0 | 0 | 0 | 0 | — | ||
| Lsp1-γ | D. yakuba | 16 | 3 | 2 | 0 | 0.0018 | 0.006 | −0.41 | 508 |
| D. santomea | 16 | 4 | 0 | 3 | 0.0024 | 0.002 | −1.55* | ||
| Ssl1 | D. yakuba | 16 | 2 | 2 | 0 | 0.0012 | 0.0051 | −0.58 | 501 |
| D. santomea | 16 | 1 | 1 | 0 | 0.0006 | 0.0025 | 0.15 | ||
| krzg | D. yakuba | 16 | 0 | — | — | 0 | 0 | — | 165 |
| D. santomea | 16 | 2 | — | — | 0.0036 | 0.0036 | −1.50* | ||
| B. Regions of nonreduced frequency of crossing over | |||||||||
| rux | D. yakuba | 16 | 28 | 20 | 7 | 0.0090 | 0.023 | −1.62* | 940 |
| D. santomea | 16 | 21 | 17 | 3 | 0.0067 | 0.02 | −1.42 | ||
| per | D. yakuba | 16 | 3 | 0 | 0 | 0.001 | 0.0013 | −1.35 | 905 |
| D. santomea | 14 | 5 | 0 | 1 | 0.0017 | 0.0019 | −1.36 | ||
| sog | D. yakuba | 16 | 25 | 23 | 2 | 0.007 | 0.024 | −0.63 | 1100 |
| D. santomea | 16 | 18 | 17 | 1 | 0.0049 | 0.018 | −0.59 | ||
| bnb | D. yakuba | 14 | 33 | 24 | 9 | 0.012 | 0.037 | −0.86 | 837 |
| D. santomea | 16 | 25 | 18 | 7 | 0.009 | 0.026 | −0.97 | ||
| Hex-A | D. yakuba | 16 | 3 | 3 | 0 | 0.0014 | 0.058 | −0.63 | 639 |
| D. santomea | 16 | 9 | 8 | 1 | 0.0042 | 0.016 | −0.67 | ||
| Rad1 | D. yakuba | 16 | 16 | 14 | 1 | 0.011 | 0.04 | −1.45 | 428 |
| D. santomea | 16 | 10 | 8 | 1 | 0.007 | 0.024 | −1.48* | ||
| RpL27A | D. yakuba | 14 | 20 | 2 | 1 | 0.0076 | 0.01 | 0.13 | 822 |
| D. santomea | 14 | 19 | 2 | 1 | 0.0073 | 0.0099 | −1.17 | ||
| salr | D. yakuba | 16 | 28 | 25 | 2 | 0.0086 | 0.038 | −0.72 | 869 |
| D. santomea | 16 | 26 | 22 | 3 | 0.0090 | 0.033 | −0.58 | ||
| Rep4 | D. yakuba | 16 | 25 | 19 | 6 | 0.0093 | 0.032 | −0.76 | 808 |
| D. santomea | 16 | 14 | 11 | 3 | 0.0052 | 0.019 | −1.61* | ||
| Sara | D. yakuba | 16 | 21 | 13 | 7 | 0.0068 | 0.02 | −0.26 | 887 |
| D. santomea | 16 | 12 | 8 | 4 | 0.0040 | 0.012 | 0.52 | ||
| Hex-C | D. yakuba | 16 | 21 | 19 | 2 | 0.011 | 0.04 | 0.65 | 552 |
| D. santomea | 16 | 13 | 12 | 1 | 0.0071 | 0.021 | −0.73 | ||
| Ngp | D. yakuba | 16 | 3 | 3 | 0 | 0.0012 | 0.005 | 0.47 | 767 |
| D. santomea | 16 | 5 | 4 | 1 | 0.0020 | 0.0067 | −0.32 | ||
| sfl | D. yakuba | 16 | 40 | 40 | 0 | 0.014 | 0.058 | −0.86 | 867 |
| D. santomea | 16 | 37 | 37 | 0 | 0.013 | 0.053 | −0.59 | ||
| Est6 | D. yakuba | 16 | 37 | 22 | 8 | 0.016 | 0.043 | 0.17 | 687 |
| D. santomea | 16 | 27 | 15 | 9 | 0.012 | 0.027 | 0.12 | ||
| Xdh | D. yakuba | 16 | 32 | 23 | 9 | 0.012 | 0.036 | −0.14 | 782 |
| D. santomea | 16 | 24 | 18 | 6 | 0.0092 | 0.028 | −1.95** | ||
| AP-50 | D. yakuba | 16 | 35 | 25 | 2 | 0.01 | 0.035 | −0.63 | 1025 |
| D. santomea | 16 | 29 | 22 | 1 | 0.0085 | 0.029 | −1.84* | ||
| Mlc1 | D. yakuba | 16 | 7 | 0 | 0 | 0.0059 | 0.0071 | −1.52 | 359 |
| D. santomea | 16 | 10 | 0 | 0 | 0.0084 | 0.01 | −1.54* | ||
P < 0.05,
P < 0.01. —, values could not be estimated because of lack of informative sites.
Sample size.
Number of polymorphic sites.
Number of synonymous (syn) and nonsynonymous (rep) polymorphic sites estimated using Nei and Gojobori (1986).
Watterson's (1975) estimate of total and silent (synonymous and noncoding) heterozygosity.
Tajima's (1989b) D-statistic. Significance was calculated with coalescent simulations without recombination.
Size of the sequenced region.
This locus does not include the coding region sequence.
DNA extraction and sequencing:
We extracted DNA from single flies using the Puregene DNA isolation kit for paraffin-embedded tissue (Gentra Systems, Minneapolis) and performed PCR amplifications using ∼25 ng of genomic DNA. To ensure the specific amplification of the genes on the Y chromosome, we performed PCR reactions using genomic DNA extracted from females as a control. PCR products were purified using the Wizard MagneSil PCR clean-up system (Promega, Madison, WI) and sequenced directly with an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Foster City, CA) after we performed cycle sequencing reactions using Big Dye 3.0 (Applied Biosystems). Both strands were sequenced. We edited sequences with the software Sequencher 3.0 (Gene Codes, Ann Arbor, MI) and aligned them using the ClustalX program (Thompson et al. 1997). Haplotypes were reconstructed (Stephens and Donnelly 2003) using the Phase 2.0 program (Stephens et al. 2001), excluding indels. We deposited all newly obtained sequences in GenBank, EMBL, and DDBJ database libraries under accession nos. AY804458–AY804777, AY804780–AY804885, and AY804888–AY805047.
Data analysis:
Basic polymorphism analyses were performed using DnaSP 4.0 (Rozas et al. 2003). To determine whether regions under study were heterogeneous in their polymorphism-to-divergence ratios, and to test whether the global frequency spectrum of polymorphisms conforms to the neutral expectation, we used the HKA program (Hey and Kliman 1993). This program obtains the significance of the observed statistics ( and D), using multilocus coalescent simulations.
and D), using multilocus coalescent simulations.
To detect the presence of significant introgression between D. yakuba and D. santomea, we used two different strategies. In regions with nonreduced frequency of crossing over we fit the data to an isolation model (i.e., a model of genic patterns based on the assumption that there is no gene exchange between species) (Wakeley and Hey 1997), following Wang et al. (1997) and using the Wakeley-Hey (WH) program. This model assumes that all variation is neutral and that population size remains constant over time, but allows for differences in selective constraints among loci (Wang et al. 1997). The parameters of the model (θ for the ancestral and descendant populations and τ, the time since physical separation) are estimated by choosing values that most closely equate expectations with observations; hence tests based on this model are conservative because all shared variation is assumed to be ancestral polymorphism. A significant excess of shared variation is the unequivocal fingerprint of introgression in D. yakuba and D. santomea, for they most likely went through a period of allopatry and came in contact secondarily recently. In particular, we calculated a χ2-statistic on the basis of the difference between expected and observed numbers of shared polymorphisms:  . We generated the null distribution of this statistic under the isolation model by multilocus coalescent simulations (Wang et al. 1997) with recombination, and we obtained the statistical significance of the observed
. We generated the null distribution of this statistic under the isolation model by multilocus coalescent simulations (Wang et al. 1997) with recombination, and we obtained the statistical significance of the observed  by comparing it to the null distribution generated. We estimated recombination between sites (ρ = 4 Ner) within DNA fragments using the maximum-composite-likelihood method implemented in the Maxhap software (Hudson 2001). The estimates obtained ranged between 0.0025 and 0.17 in D. yakuba and between 0.0025 and 0.5 in D. santomea.
by comparing it to the null distribution generated. We estimated recombination between sites (ρ = 4 Ner) within DNA fragments using the maximum-composite-likelihood method implemented in the Maxhap software (Hudson 2001). The estimates obtained ranged between 0.0025 and 0.17 in D. yakuba and between 0.0025 and 0.5 in D. santomea.
Loci on the tips and near centromeres of chromosomes show a reduced frequency of crossing over (Figure 1) in species of the D. melanogaster subgroup (Ashburner 1989; True et al. 1996). This reduction, usually associated with decreased levels of polymorphism, compromises the detection of shared variation. Thus in these regions and also in mtDNA and Y chromosome genes, our strategy to detect introgression was based on comparing divergence estimates between D. yakuba and D. santomea with those between D. mauritiana and D. sechellia. We derived these estimates for synonymous and nonsynonymous sites (Ks and Ka, respectively), as well as confidence intervals, using the program K-Estimator 5.5 (Comeron 1999). To estimate overall divergence between species pairs, we constructed a concatenated sequence for genes on each chromosome separately and also for all genes taken together as a single unit. To obtain maximum-likelihood estimates of the population migration rates we fit the polymorphism and divergence data on genes of reduced crossing over to an isolation with migration (IM) model (Hey and Nielsen 2004). This Markov chain Monte Carlo method assumes no intragenic recombination and yields estimates of multiple population parameters. Convergence of the Markov chain simulations was assessed by comparing the results and using different starting points.
RESULTS
Intraspecific nucleotide variation:
We collected polymorphism data in D. yakuba and D. santomea from 29 regions: 28 nuclear and 1 mitochondrial (Table 2). D. yakuba shows significantly higher levels of within-species variation than does D. santomea: the weighted average values of Watterson's (1975) estimator of 4Neμ (where Ne is the effective population size and μ is the neutral mutation rate) per site (θ) are 0.0055 for D. yakuba and 0.0044 for D. santomea (Wilcoxon signed rank test using individual genes; Z = −2.22, P = 0.026). This result is consistent with D. santomea having a smaller Ne, although this reduction of only 20% contrasts with a large difference in current population size inferred from the geographical distribution of both species.
In both species, silent variation (synonymous and noncoding) for genes on the X chromosome tends to be smaller than that for genes on the autosomes. The difference is close to the neutral expectation (a 25% reduction for the X chromosome compared to autosomes) based on their difference in Ne: θautosomal = 0.025 vs. θX = 0.015 for D. yakuba, and θautosomal = 0.018 vs. θX = 0.012 for D. santomea. Polymorphism in the mitochondrial region ND5–ND4 in D. yakuba and D. santomea is similar to that seen in D. melanogaster (Rand et al. 1994; Rand and Kann 1996). Consistent with previously published data from D. melanogaster (Zurovcova and Eanes 1999), in both D. santomea and D. yakuba we see extremely low levels of variation on the Y chromosome.
We also compared nucleotide variation between allopatric and sympatric populations of D. yakuba and D. santomea for all genic regions under study. If introgression has occurred recently in sympatric populations, one expects a certain degree of genetic differentiation between sympatric and allopatric populations of the different species. K*ST values (Hudson et al. 1992), a measure of population substructure, are consistently close to 0. Permutation tests (Hudson et al. 1992) show that only 5 of the 29 regions have significantly nonzero values of K*ST (salr and Kr in D. yakuba and bnb, Est6, and sara in D. santomea), and none of these remain significant after Bonferroni correction for multiple tests (Rice 1989). Even for these 5 regions, differences between allopatric and sympatric populations account only for 2.3–5.8% of the total molecular variance (Excoffier et al. 1992). Overall, our results show no genetic difference between sympatric and allopatric populations of D. yakuba and D. santomea on São Tomé Island. Consistently, the fraction of shared/total polymorphisms is similar for allopatric and sympatric flies (7.7 and 7.9%, respectively).
Tests for neutrality:
Under the neutral theory of molecular evolution, regions of the genome that evolve rapidly should also show high levels of intraspecific variation (Kimura 1983), a prediction that can be evaluated with the HKA test (Hudson et al. 1987). For data from several loci one can test the heterogeneity of the polymorphism-to-divergence ratio across all the regions under study (Hey and Kliman 1993). When applied to the 29 loci with polymorphism data in both D. yakuba and D. santomea, this test shows a significant departure from the neutral expectation ( ; see materials and methods for details). This departure is also observed when the five regions with significant population structure are excluded
; see materials and methods for details). This departure is also observed when the five regions with significant population structure are excluded  , and it is due primarily to regions located on tips and near centromeres of chromosomes. If these regions are excluded from the analysis, the data fit the neutral expectations
, and it is due primarily to regions located on tips and near centromeres of chromosomes. If these regions are excluded from the analysis, the data fit the neutral expectations  .
.
We also used Tajima's D (Tajima 1989b) to determine whether the frequency of mutations in our sample is consistent with neutral expectations. Most genes in our data set show negative D-values in both species (implying an excess of low-frequency variants over that expected under the neutral theory), although none of these values are statistically significant after multiple test correction. However, a multilocus analysis (Hey and Kliman 1993) shows that in both species the mean D-value among loci departs from neutral expectations in a negative direction (D = −0.49, P = 0.009 for D. yakuba and D = −0.89, P < 0.0001 for D. santomea). We observe the same pattern after excluding regions that have a reduced frequency of crossing over (D = −0.59, P = 0.009 for D. yakuba, and D = −0.95, P < 0.0001 for D. santomea) or regions with significant population structure (D = −0.53, P = 0.007 for D. yakuba, and D = −1.03, P < 0.0001 for D. santomea). Significant negative values of D imply either purifying selection or population expansion (Tajima 1989a,b).
Analysis of introgression:
Speciation can be thought of as the fragmentation of an ancestral population into two descendant populations that, in time, acquire reproductive isolation (i.e., genetic fixed differences). If the two incipient species exchange genes, the accumulation of fixed differences is retarded and shared polymorphisms are introduced. Yet this same pattern is also expected in isolated incipient species, due to shared variants inherited from their common ancestor, particularly if speciation did not involve a strong bottleneck. There is also a third source of shared variation: independent parallel mutations, which may be a special problem in species that are very polymorphic.
To test for independent mutations as a factor that may mimic introgression, we calculated the expected number of recurrent mutations using a hypergeometric distribution conditioning on the number of polymorphisms in each species and the number of sites under analysis (Clark 1997; Kliman et al. 2000). In D. yakuba and D. santomea, 56 of the 719 polymorphisms are shared (Table 3), while the expectations are just 5.85 under a model in which variation is evenly distributed along all sites, and slightly more—13.01—when only silent sites are considered. The number of observed shared polymorphisms substantially exceeds both of these expectations (P < 1 × 10−6). Therefore a significant fraction of shared variation cannot be explained by parallel mutation and must be accounted for by either introgression or common ancestry.
TABLE 3.
Shared and fixed variation between D. yakuba and D. santomea
| Locus | SSa | Fb | Sx1c | Sx2d | Locus | SSa | Fb | Sx1c | Sx2d |
|---|---|---|---|---|---|---|---|---|---|
| A. Regions of reduced frequency of crossing over | |||||||||
| ND5/ND4 | 2 (0.01) | 0 | 6 | 2 | His3 | 0 (0) | 6 | 3 | 0 |
| Y chr.e | 0 (0) | 8 | 2 | 3 | Kr | 0 (0) | 6 | 3 | 0 |
| y | 0 (0) | 0 | 6 | 0 | Lsp1-γ | 0 (0.02) | 4 | 3 | 4 |
| su(f) | 0 (0) | 7 | 1 | 0 | Ssl1 | 0 (0) | 6 | 2 | 1 |
| l(2)gl | 0 (0) | 1 | 1 | 3 | krz | 0 (0) | 3 | 0 | 2 |
| B. Regions of nonreduced frequency of crossing over | |||||||||
| rux | 3 (0.51) | 22 | 25 | 19 | Sara | 0 (0.27) | 2 | 20 | 12 |
| per | 0 (0.02) | 13 | 3 | 5 | Hex-C | 2 (0.38) | 0 | 19 | 11 |
| sog | 2 (0.33) | 1 | 23 | 16 | Ngp | 0 (0.02) | 3 | 3 | 5 |
| bnb | 5 (0.67) | 2 | 28 | 20 | sfl | 12 (0.81) | 2 | 28 | 25 |
| Hex-A | 0 (0.04) | 3 | 3 | 9 | Est6 | 5 (0.99) | 5 | 31 | 22 |
| Rad1 | 2 (0.26) | 1 | 14 | 8 | Xdh | 3 (0.78) | 1 | 29 | 21 |
| RpL27A | 0 (0.46) | 2 | 20 | 19 | AP-50 | 7 (0.60) | 4 | 28 | 22 |
| salr | 10 (0.23) | 0 | 13 | 14 | Mlc1 | 2 (0.11) | 3 | 5 | 8 |
| Rep4 | 1 (0.39) | 4 | 24 | 13 | |||||
The number of polymorphisms expected by recurrent independent mutations is indicated in parentheses (Clark 1997).
Shared polymorphisms.
Fixed differences.
Exclusive polymorphisms in D. yakuba.
Exclusive polymorphisms in D. santomea.
Y chromosome genes DhcYh3, CG17629, and Pp1Y1 were pooled.
To claim shared variation as evidence of introgression we ought to test it against a model that takes into account possible ancestral polymorphism. This approach based on detecting an excess of shared variation, however, is less informative in regions where polymorphism is severely reduced. In Drosophila, as in many other eukaryotes, regions of reduced frequency of crossing over show a strong reduction of intraspecific variation because of recurrent selection (Aguadé et al. 1989; Stephan and Langley 1989; Berry et al. 1991; Begun and Aquadro 1992; Langley et al. 1993; Dvorak et al. 1998; Kraft et al. 1998; Nachman et al. 1998; Przeworski et al. 2000). Thus to investigate introgression between D. yakuba and D. santomea we analyzed separately genes of nonreduced and reduced crossing over.
Regions of nonreduced crossing over:
To determine whether the number of shared polymorphisms exceeds the expectations estimated using the isolation model proposed by Wakeley and Hey (1997) we used the  -statistic (see materials and methods). We performed two sets of tests. In the first, we contrast
-statistic (see materials and methods). We performed two sets of tests. In the first, we contrast  for each locus against the expected distribution of this statistic under the isolation model, and in the second we add the
for each locus against the expected distribution of this statistic under the isolation model, and in the second we add the  -values of all loci to assess whether the observed variance among loci differs from that expected under the isolation model. Tests of individual loci show a highly significant excess of shared polymorphisms in the salr region
-values of all loci to assess whether the observed variance among loci differs from that expected under the isolation model. Tests of individual loci show a highly significant excess of shared polymorphisms in the salr region  . The same tendency is observed for the sfl gene
. The same tendency is observed for the sfl gene  on the third chromosome, but this is not significant after the Bonferroni correction. salr is on the second chromosome and, as a consequence, the isolation model is also rejected when we test the whole chromosome
on the third chromosome, but this is not significant after the Bonferroni correction. salr is on the second chromosome and, as a consequence, the isolation model is also rejected when we test the whole chromosome  . This result should not be interpreted as evidence for whole-chromosome exchange between D. yakuba and D. santomea, but as an indication that gene flow involving this chromosome is heterogeneous, with at least one locus having a number of shared polymorphisms that is not consonant with the background level. Despite the significant departure from the isolation model for salr and the second chromosome, the overall patterns of variation among all regions of nonreduced frequency of crossing over are compatible with the isolation model (Table 5), which suggests that introgression is not pervasive.
. This result should not be interpreted as evidence for whole-chromosome exchange between D. yakuba and D. santomea, but as an indication that gene flow involving this chromosome is heterogeneous, with at least one locus having a number of shared polymorphisms that is not consonant with the background level. Despite the significant departure from the isolation model for salr and the second chromosome, the overall patterns of variation among all regions of nonreduced frequency of crossing over are compatible with the isolation model (Table 5), which suggests that introgression is not pervasive.
TABLE 5.
Testing the isolation model in D. yakuba and D. santomea
| θ1 | θ2 | θA |  |
PSSa |  |
PTa | WWH | PWWHa | |
|---|---|---|---|---|---|---|---|---|---|
| X | 18.93 | 15.48 | 109.59 | 3.77 | 0.70 | 54.24 | 0.24 | 26 | 0.16 |
| 0.12–38.01 | 0.12–32.41 | 54.98–165.82 | |||||||
| 2 | 34.37 | 23.69 | 46.98 | 29.56 | 0.0075 | 50.97 | 0.034 | 14 | 0.063 |
| 21.00–53.07 | 15.59–34.62 | 24.54–69.47 | |||||||
| 3 | 28.26 | 22.75 | 75.63 | 4.91 | 0.80 | 14.40 | 0.97 | 14 | 0.73 |
| 9.52–52.80 | 7.92–42.60 | 40.56–120.3 | |||||||
| Totalb | 76.91 | 61.15 | 232.37 | 33.98 | 0.46 | 134.12 | 0.4 | 34 | 0.12 |
| 50.29–106.76 | 40.76–83.68 | 169.42–308.15 | |||||||
| Totalc | 78.72 | 62.54 | 321.30 | 4135.15 | <0.0001 | 4382.35 | <0.0001 | 34 | 0.21 |
| 45.65–113.24 | 37.22–87.60 | 221.64–411.42 |
Estimated values of parameters of the isolation model are shown (Wakeley and Hey 1997) as performed in Wang et al. (1997) with 95% confidence intervals based on coalescent simulations.
Fractions of simulations with values of  (see text), χT2 (see Kliman et al. 2000 for details), and WWH (see Wang et al. 1997 for details) statistics equal to or more extreme than the values observed.
(see text), χT2 (see Kliman et al. 2000 for details), and WWH (see Wang et al. 1997 for details) statistics equal to or more extreme than the values observed.
Only genes in regions of nonreduced crossing over (17 genes).
Regions of nonreduced and reduced crossing over (mtDNA and Y chromosome included, 29 genes).
Regions of reduced crossing over:
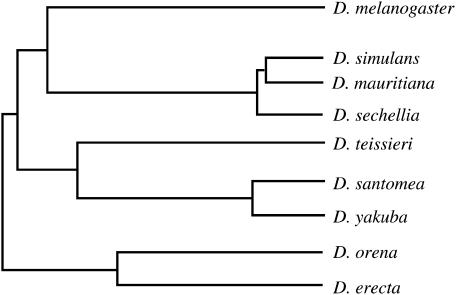
Loci on the tips and near centromeres of chromosomes show a severe reduction of polymorphism in D. yakuba and D. santomea (Mann-Whitney test; Z = −3.76, P = 0.0002 for D. yakuba and Z = −4.31, P < 0.0001 for D. santomea). This is consistent with these regions having a reduced frequency of crossing over, but makes difficult the detection of introgression through shared variation. Divergence, however, can be used to examine gene flow, for one of the consequences of introgression is the reduction of interspecific differences in the regions being exchanged. Such a reduction is also expected under strong selective constraints. To distinguish between these two possibilities, we compared estimates of genetic divergence between D. yakuba and D. santomea with estimates from a pair of allopatric species in the same group: species in which gene flow is very unlikely. These species are D. mauritiana and D. sechellia. Each is endemic to an Indian Ocean island very far from the other (Mauritius and the Seychelles, respectively), and these well-studied species almost certainly arose after the independent colonization of the islands by their common ancestor with the mainland African species D. simulans (Figure 2).
Figure 2.
Schematic of the phylogenetic relationships among the nine species of the D. melanogaster subgroup (Kliman et al. 2000; Lachaise et al. 2000; Parsch 2003).
Ideally, one would compare species pairs separated by similar genetic distances. (Using this criterion assumes that introgression is not so pervasive as to distort the genetic differences at all loci used to calculate divergence times.) To check that this was so for the species pairs used in our test, we therefore estimated divergence between D. yakuba and D. santomea using newly obtained sequences of 45 nuclear genes (Table 1). We also estimated divergence between D. mauritiana and D. sechellia, using published sequences of 25 genes (supplementary Table S1 at http://www.genetics.org/supplemental/). For D. yakuba and D. santomea, the estimated number of nonsynonymous substitutions per site, Ka, is 0.0029 [95% confidence intervals (C.I.) 0.0022–0.0035], while divergence at synonymous sites, Ks, is 0.044 (C.I. 0.039–0.048). For D. mauritiana and D. sechellia, estimates for Ka and Ks are 0.0083 (C.I. 0.0069–0.0097) and 0.047 (C.I. 0.04–0.053), respectively. Clearly the Ks values, which are critical in determining species age, are very similar for these species pairs. Thus, comparative analysis of interspecific divergence between these two pairs of species allows us to determine what genes—if any—are able to cross the species boundary between D. yakuba and D. santomea. Such introgression is revealed by a reduction of divergence compared to that seen between truly allopatric species.
We compared estimates of divergence in the 12 regions of reduced crossing over for D. yakuba and D. santomea with estimates in the same regions between D. mauritiana and D. sechellia (Table 4). MtDNA and genes on the Y chromosome have, a priori, similar abilities to reveal the genetic history of species, as they both experience an equivalent reduction in Ne compared to that in autosomal loci. However, mtDNA and the Y chromosome show strikingly different patterns of genetic divergence between D. yakuba and D. santomea. The number of substitutions in the Y chromosome is similar for the D. yakuba/D. santomea and D. mauritiana/D. sechellia comparisons (Table 4). In contrast, D. yakuba/D. santomea show a >60-fold reduction in mitochondrial divergence compared to that in D. mauritiana/D. sechellia (K = 0.0006 vs. K = 0.039, P < 0.0001 and Table 4). The reduction is >50-fold if only synonymous sites are considered. These results suggest that introgression of mtDNA has occurred between D. yakuba and D. santomea.
TABLE 4.
Substitutions between D. yakuba-D. santomea and D. mauritiana-D. sechellia in regions with reduced frequency of crossing over
| Locus | Locationa | D. yakuba- D. santomeab | D. mauritiana- D. sechellia | Size (bp) |
|---|---|---|---|---|
| ND4-ND5c | mtDNA | 1 | 62 | 1658 |
| Y chr.d | Y | 7 | 6 | 2155 |
| ye | X | 1 | 6 | 1041 |
| su(f) | X | 7 | 0 | 717 |
| l(2)gl | 2 | 9 | 9 | 903 |
| His3 | 2 | 5 | 11 | 811 |
| Kr | 2 | 10 | 9 | 1329 |
| Lsp1-γ | 3 | 7 | 4 | 659 |
| Ssl1 | 3 | 15 | 7 | 754 |
| krz | 3 | 2 | 4 | 207 |
| eyf | 4 | 6 | 5 | 918 |
In D. melanogaster.
Number of substitutions based on single sequence comparison (D. yakuba Taï18 and D. santomea STO.4).
D. mauritiana and D. sechellia sequences were obtained from GenBank (accession nos. AF200830 and AF200832).
Includes Dhc-Yh3 and CG17629, but not Pp1Y1 because we could not amplify it in D. mauritiana and D. sechellia. D. mauritiana and D. sechellia sequences for Dhc-Yh3 were obtained from GenBank (accession nos. AF136265 and AF136264).
The D. mauritiana sequence was obtained from GenBank (accession no. AJ300669).
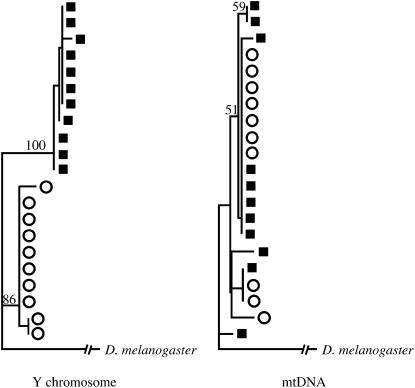
Analysis of population differentiation (Hudson et al. 1992) indicates that the mtDNA of D. yakuba is not genetically different from that of D. santomea (K*ST = −0.01, P = 0.54). Indeed, 9 out of 10 lines analyzed in D. santomea have the same mtDNA haplotype as do 5 out of 6 D. yakuba lines collected in São Tomé. In addition, mtDNA haplotypes of D. yakuba collected in mainland Africa are significantly different from those of D. yakuba and D. santomea lines collected on the island (K*ST = 0.072, P = 0.02), another hint of mtDNA introgression. As this observation implies, mainland and island strains of D. yakuba are also genetically different (K*ST = 0.88, P = 0.044). Consequently, gene genealogies based on mtDNA show alleles of D. santomea clustering with alleles of D. yakuba, a pattern that stands in strong contrast to that seen for the Y chromosome (Figure 3).
Figure 3.
Gene genealogies from the Y chromosome and mtDNA. The reconstruction was done using the neighbor-joining algorithm (Saitou and Nei 1987). The numbers above the branches are bootstrap values based on 1000 replicates (>50%). Solid squares, D. yakuba; open circles, D. santomea.
Lachaise et al. (2000) also reported a high level of similarity among sequences of the mitochondrial gene cytochrome b in D. yakuba, D. santomea, and D. teissieri (Monnerot et al. 1990). We investigated whether this similarity could reflect an introgression of mtDNA between more than two species by examining synonymous divergence at nuclear and mtDNA genes among these species (supplementary Table S2 at http://www.genetics.org/supplemental/). Synonymous divergence at nuclear genes gives us an estimate of the times of these species splits, which we can then compare to divergence times estimated from mtDNA and Y chromosome genes. If there is introgression of mtDNA, one expects reduced divergence times for mtDNA compared to those for nuclear genes.
We sequenced the ND5–ND4 region and two genes in the Y chromosome (Dhc-Yh3 and CG17629; we could not amplify Pp1Y1 in D. teissieri) in D. teissieri. In addition, we combined our data with sequences for 26 nuclear genes in D. teissieri and D. yakuba taken from GenBank. The overall estimate for synonymous and nonsynonymous divergence between these 26 nuclear genes for D. yakuba and D. teissieri is Ks = 0.087 (C.I. 0.079–0.094) and Ka = 0.017 (C.I. 0.015–0.019), respectively. Ks for the mitochondrial ND5–ND4 region between D. yakuba and D. teissieri is 0.011, the lowest value among all genes analyzed. Therefore, our results are consistent with a reduced mtDNA divergence between D. yakuba and D. teissieri compared to that in nuclear genes, but we also find fixed differences between the pair D. yakuba/D. santomea on one hand and D. teissieri on the other in the regions studied (4 for the ND5–ND4 region of mtDNA, 32 for the Y chromosome). These results suggest that while there was ancient introgression of mtDNA between the ancestor of D. teissieri and that of D. santomea/D. yakuba, there has also been a more recent introgression of mtDNA between D. yakuba and D. santomea, presumably after D. yakuba experienced secondary contact with D. santomea on São Tomé.
For the remaining genes in regions of reduced crossing over, exon 2 of yellow is the only one showing significantly less divergence between D. yakuba and D. santomea than between D. mauritiana and D. sechellia after correcting for multiple tests (Rice 1989) (K = 0.001 vs. K = 0.0058, P < 0.0001). In fact, D. santomea shows no variation in this region: all sequences are identical to that of the most common haplotype in D. yakuba. Moreover, the multilocus HKA test (Hey and Kliman 1993) shows a significant heterogeneity of the polymorphism-to-divergence ratio across these eight regions when the Y chromosome and mtDNA loci are excluded  . This heterogeneity is not observed when only the yellow region is excluded from the analysis
. This heterogeneity is not observed when only the yellow region is excluded from the analysis  . We therefore suggest that the lack of fixed differences in the yellow gene between D. yakuba and D. santomea is the main cause of the heterogeneity among these regions having reduced crossing over. The similarity between these species in the yellow region, however, is not observed in a fragment located ∼5 kb upstream from the start codon (data not shown). Indeed, we obtained the sequence for this additional 0.7-kb-long fragment in the same flies for which the second exon of yellow was studied, and found eight fixed differences between D. yakuba and D. santomea and no shared polymorphism (see discussion). The introgression in the yellow region may reflect its lack of effect on hybrid sterility (see discussion).
. We therefore suggest that the lack of fixed differences in the yellow gene between D. yakuba and D. santomea is the main cause of the heterogeneity among these regions having reduced crossing over. The similarity between these species in the yellow region, however, is not observed in a fragment located ∼5 kb upstream from the start codon (data not shown). Indeed, we obtained the sequence for this additional 0.7-kb-long fragment in the same flies for which the second exon of yellow was studied, and found eight fixed differences between D. yakuba and D. santomea and no shared polymorphism (see discussion). The introgression in the yellow region may reflect its lack of effect on hybrid sterility (see discussion).
Finally, we obtained maximum-likelihood estimates (MLEs) of the population migration rates between D. yakuba and D. santomea by fitting polymorphism and divergence data on genes with reduced frequency of crossing over to the recently proposed IM model (Hey and Nielsen 2004). MLEs of population migration rates (M; M = 2 Nem, where m is the migration rate) are m1 = 0.0008 and m2 = 0.062 for D. santomea to D. yakuba and for D. yakuba to D. santomea, respectively. Ninety-five percent confidence intervals of m2 rule out a scenario with no introgression of genes from D. yakuba to D. santomea.
Autosomal vs. sex-linked genes:
The study of hybrid male sterility in D. yakuba and D. santomea revealed at least three male “sterility genes” that map to the X chromosome, as well as a significant effect of foreign Y chromosomes on sterility (Coyne et al. 2004). Thus, all else being equal, the movement of sex chromosomes across the hybrid zone should be limited. We therefore examined whether the Y and X chromosomes show less introgression between these species than do the autosomes. For the Y chromosome, none of the three genes studied shows shared variation and all show fixed interspecific differences (Table 3). Thus there is no evidence of recent gene flow between D. yakuba and D. santomea involving this chromosome. This result is, of course, consistent with the expectation that the Y chromosome is the least likely to introgress because it not only causes sterility by itself, but also is restricted to males, which are completely sterile as F1 hybrids and largely sterile in backcrosses (Coyne et al. 2004).
To compare the amount of introgression between the X chromosome and autosomes, we calculated the number of shared polymorphisms and fixed differences between D. yakuba and D. santomea for these chromosomes. We used only variation in regions with “normal” frequencies of crossing over to avoid artificially distorting the polymorphism-to-divergence ratio differentially for X chromosomes and autosomes. The ratio of shared to fixed differences is significantly lower for genes on the X chromosome than for those on the autosomes (10/41 for the X chromosome and 44/27 for the autosomes, G-test, G = 22.72, P < 0.0001). While this result is consistent with reduced introgression of the X chromosome, there is an alternative explanation: the difference in Ne between X chromosomes and autosomes is also expected to reduce both θ and the number of shared polymorphisms and to increase the number of fixed differences for X-linked compared to autosomal regions. To properly judge the likelihood of introgression, we must correct for this difference in Ne. To do so, we estimated the number of shared and fixed differences for the X chromosome and autosomes under the isolation model (9.47 and 28.79, respectively, for the X chromosome, and 44.52 and 39.20, respectively, for the autosomes). The deviation of the observed values from the expectations is estimated with a χ2-statistic, and the statistical significance is obtained by comparing the observed χ2, 8.98, with a null distribution of this statistic obtained by multilocus coalescent simulations. Note that in very closely related species, the variance of the outcome of the coalescent process has a substantial effect on the number of fixed differences. Although we observe a higher than expected number of fixed differences for the X chromosome (and a deficit of fixed differences on the autosomes), the deviation from expectation is not statistically significant (P = 0.18). Therefore, although the difference between the X chromosome and the autosomes is in the direction indicating less introgression of the former between D. yakuba and D. santomea, this difference is not significant.
DISCUSSION
We examined intra- and interspecific variation for 29 randomly selected loci regions of D. yakuba and D. santomea. Overall, the data are not compatible with an isolation model that assumes no gene flow between these species after they began to diverge (P = 0.0001, Table 5). Therefore, the genomes of these entities have not remained completely distinct, almost certainly because of past hybridization. Nevertheless, this gene flow has not been extensive: only 3 of the 29 regions (mtDNA and the nuclear regions containing the yellow and salr loci) show statistically significant evidence for introgression. Thus these species do not form a “hybrid swarm” on São Tomé in which there is pervasive introgression in much of the genome, while disruptive selection maintains distinctness for only a few traits (e.g., pigmentation and male genitalia). However, we must bear in mind that the introgression we are able to detect today is unlikely to reflect very recent gene flow, for neutral mutations require substantial time to reach detectable frequencies in a population (Kimura and Ohta 1972; Nei and Feldman 1972).
Introgression in regions of nonreduced crossing over:
The salr gene, located in 2L, shows evidence of introgression between D. yakuba and D. santomea. Its level of shared polymorphism and the absence of fixed differences between species stand out against a chromosomal background practically devoid of shared variation. There is a strong reduction of the ratio of shared-to-exclusive polymorphisms on the right arm of the second chromosome, which has the most inversion polymorphism in D. yakuba, compared to that on chromosome 3 (2/70 vs. 29/219, G = 6.4, P = 0.011). These results suggest that gene flow is particularly restricted in 2R, an observation consistent with the hypothesis that genes contributing to reproductive isolation accumulate faster in chromosomal inversions than in colinear regions of the genome (Rieseberg et al. 1995; Noor et al. 2001; Navarro and Barton 2003). In agreement with this proposal, Machado et al. (2002) reported that regions that are distinct between D. pseudoobscura and D. persimilis are also located in chromosomal inversions. Clearly the effect of inversions on limiting gene flow is greatest if they are fixed between species, but polymorphic inversions may also contribute, to a lesser degree, to restricting gene flow. The combination of shared variation for salr on 2L and the absence of shared variation on 2R leads to the result that patterns of variation in the entire second chromosome are incompatible with the isolation model—a result not seen in the X or third chromosomes.
The isolation model is not rejected when all genes in regions of nonreduced crossing over are considered. The estimated values of the parameter θA of the isolation model (Table 5), however, suggest that the ancestral species of D. yakuba and D. santomea had an effective population size larger than that of either of the derived species, a result that is inconsistent with the observed negative values of D (i.e., population expansion). Thus, it is conceivable that the degree of introgression between D. yakuba and D. santomea may have been underestimated.
Introgression in regions with reduced crossing over:
Divergence between D. yakuba and D. santomea for the mitochondrial region ND5–ND4 is strongly reduced compared to both the rest of the genes in these species' genomes and the divergence between D. mauritiana and D. sechellia. This reduction is even more conspicuous when we consider that mitochondrial genes evolve 4.5–9 times faster than nuclear genes (Moriyama and Powell 1997) at synonymous sites and is a sound indication that D. yakuba and D. santomea have exchanged mtDNA. In addition, the D. yakuba and D. santomea mtDNA haplotypes found on São Tomé are more similar to each other than to mtDNA haplotypes found on the African mainland. We therefore conclude that the lack of fixed differences in the ND5–ND4 region between D. yakuba and D. santomea, together with the observation of shared haplotypes on the island, strongly suggests that these species have exchanged mitochondrial genomes.
There is ample evidence from many groups that mtDNA introgresses between species more frequently than does nuclear DNA (Smith 1992; Ferris et al. 1993; Bernatchez et al. 1995; Taylor and McPhail 2000; Shaw 2002; Ballard and Whitlock 2004). The reason for this pattern is not yet understood, but Hudson and Coyne (2002) and Coyne and Orr (2004) suggest two reasons. First, mitochondrial loci appear to have primarily “housekeeping” functions, such as production of tRNAs and enzymes used in respiration. Selection on such loci may be largely divorced from the external (but not the internal) environment, and therefore mitochondria from one species may function relatively well on the genetic background of a closely related species. In addition, even “neutral” nuclear DNA may often fail to introgress during hybridization because it is linked to other genes that are divergently selected between species.
Because mainland African populations of D. yakuba can be thought of as an outgroup, it is very likely that the direction of introgression of mtDNA has been from D. yakuba into D. santomea. (The alternative direction of introgression would require the unlikely scenario that D. santomea mtDNA introgressed into D. yakuba on São Tomé, and this mtDNA subsequently spread throughout D. yakuba on the African mainland.) This result is consonant with laboratory studies showing that, while D. yakuba and D. santomea produce hybrids in both directions, interspecific matings occur much more frequently between D. yakuba females and D. santomea males than vice versa (Coyne et al. 2002).
The mtDNA haplotype found in a single strain in D. santomea collected on the southwest part of São Tomé is identical to one D. santomea haplotype (though not the most frequent) detected on Pico de São Tomé, suggesting that D. yakuba haplotypes may be fixed on the entire island. The similarity of mtDNA between D. yakuba and D. santomea may have resulted from either genetic drift or selection following introgression. The presence of some polymorphisms in the mtDNA of the D. yakuba and D. santomea lines from São Tomé Island, as well as the detection of shared variation on this organelle, points to a neutral explanation. On the other hand, given the average time to fixation for neutral mutations (Kimura 1983), and the relatively short divergence time between these species, it remains plausible that selection may have played some role in the invasion of the D. yakuba mtDNA through a “transspecies-selective sweep” (Hilton et al. 1994; Stephan et al. 1998; Machado and Hey 2003). Under this scenario, an ancient selective sweep of D. yakuba mtDNA through both species was followed by the appearance of new mutations on mtDNA that later introgressed between the species. It is unlikely that this mitochondrial sweep could have been associated to the spread of a Wolbachia infection, as proposed in D. simulans (Turelli and Hoffmann 1995; Ballard 2004), because D. yakuba and D. santomea do not show cytoplasmic incompatibility when infected by natural strains of Wolbachia (Zabalou et al. 2004).
As with mtDNA, divergence in the yellow region between D. yakuba and D. santomea is reduced compared to that seen between D. mauritiana and D. sechellia. That this disparity is also due to introgression rather than to selection on amino acids is supported by the observed fivefold difference in Ks (Ks = 0.0029 for D. yakuba-D. santomea and Ks = 0.015 for D. mauritiana-D. sechellia). In addition, all D. santomea yellow haplotypes are identical to the most frequent haplotype seen in the D. yakuba sample.
Apart from introgression, there is one other hypothesis that could explain the pattern observed in yellow: stronger selective constraints at synonymous sites at yellow in D. yakuba and D. santomea than in the D. simulans clade. Indeed, Takano-Shimizu (1999) has suggested stronger selective constraints at yellow synonymous sites in the D. yakuba lineage compared to the D. melanogaster lineage (using D. orena as outgroup; Figure 2) on the basis of patterns of synonymous codon usage. However, there is no reduction in the number of synonymous substitutions in the D. yakuba lineage compared to the D. melanogaster lineage (Takano-Shimizu 1999). Moreover, synonymous polymorphism at yellow in D. yakuba is not drastically reduced compared to that in the other nuclear regions studied, with 14 of the 29 regions analyzed showing lower polymorphism than this locus (10/12 genes in regions of reduced crossing over and 4/12 in regions of nonreduced crossing over). Therefore, we conclude that the lack of divergence in the yellow locus between D. yakuba and D. santomea largely reflects introgression. This is also consistent with evidence that this region apparently does not contain genes affecting male sterility in D. yakuba/D. santomea hybrids (Coyne et al. 2004). However, we do not detect introgression in a region only 5 kb upstream from the start codon of yellow. It has been suggested that the telomere end of the D. yakuba X chromosome shows a substantial increase in recombination (∼14-fold higher) compared to that of the D. melanogaster X chromosome (Takano-Shimizu 1999). Thus, we tentatively propose that introgression is restricted to the coding region of the yellow gene, while a 5′ flanking region remains distinct. This in turn suggests that introgression can be a quite localized phenomenon on the chromosome (Wang et al. 1999; Ting et al. 2000; Takahashi et al. 2001).
Autosomal vs. sex-linked genes:
We also tested the hypothesis that there is less interspecific introgression of genes on the X chromosome than on the autosomes because of the large effect of the X chromosome on hybrid male sterility in D. yakuba and D. santomea (Coyne et al. 2004). If the X chromosome contributes a disproportionate effect on hybrid male sterility and inviability (Charlesworth et al. 1987; Coyne and Orr 1989), all else being equal, there should be less introgression for the X chromosome than for autosomes. The numbers of fixed differences between these species for the X chromosome and autosomes are higher and lower, respectively, than the expectations under the isolation model, but these differences are not statistically significant when tested by coalescent simulation. These differences, however, are in the direction expected if the X chromosome shows less introgression, and it is possible that in such closely related species the large variance of the coalescent process denies us the statistical power to demonstrate this.
Alternatively, there are two other explanations for a lack of difference in introgression of X-linked vs. autosomal genes. First, the large effect of the X chromosome may reflect not a difference in the number of genes causing sterility, but merely its expression of recessively acting sterility genes in males—the so-called “dominance theory,” for which there is substantial evidence (Coyne and Orr 2004). If the density of sterility genes is similar on all chromosomes, it is thus possible that “X effects” in males caused by recessivity alone (rather than a higher density of sterility genes) would not lead to a reduced rate of introgression. Theoretical work is needed to explore this possibility. However, recent experiments in D. simulans and D. mauritiana show that X-chromosomal sterility genes are not only partially recessive, but also more numerous than those on autosomes (Tao et al. 2003). If this were also the case in D. yakuba and D. santomea, one would indeed expect less introgression of X-linked vs. autosomal genes.
Alternatively, reduced introgression of the X chromosome caused by its larger effect on hybrid sterility may be balanced by the accumulation of autosomal genes contributing to other reproductive barriers, such as sexual or habitat isolation. Theoretical work by Orr and Betancourt (2001)(Orr and Betancourt 2001), for example, shows that when adaptation to a sudden change in environmental conditions is achieved through selection of existing genetic variation at initial mutation/selection equilibrium, genes in the X chromosome are expected to evolve less rapidly than those in autosomes.
Acknowledgments
We thank D. Charlesworth and two anonymous reviewers for comments and suggestions. We are also grateful to J. M. Comeron for helping us with different programs and to S. Elwyn and S. Powers for their assistance in the laboratory. We thank A. Chang for collecting flies, S. Sousa (Conservation et utilization rationale des ECOsystèmes Forestiers D'Afrique Centrale, ECOFAC) for assistance in the field, and the Government of São Tomé and Principé for allowing us to work in the Obo National Park. This work was supported by National Institutes of Health grant GM58260 to J. A. Coyne.
References
- Aguadé, M., N. Miyashita and C. H. Langley, 1989. Reduced variation in the yellow-achaete-scute region in natural populations of Drosophila melanogaster. Genetics 122: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, M. L., 1997. Natural Hybridization and Evolution. Oxford University Press, Oxford.
- Ashburner, M., 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Ballard, J. W., 2004. Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Mol. Biol. Evol. 21: 428–442. [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O., and M. C. Whitlock, 2004. The incomplete natural history of mitochondria. Mol. Ecol. 13: 729–744. [DOI] [PubMed] [Google Scholar]
- Begun, D. J., and C. F. Aquadro, 1992. Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356: 519–520. [DOI] [PubMed] [Google Scholar]
- Bernatchez, L., L. Glemete, C. C. Wilson and R. G. Danzmann, 1995. Introgression and fixation of Arctic char (Salvenlinus alpinus) mitochondrial genome in an allopatric population of brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 52: 179–185. [Google Scholar]
- Berry, A. J., J. W. Ajioka and M. Kreitman, 1991. Lack of polymorphism on the Drosophila fourth chromosome resulting from selection. Genetics 129: 1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton, R. E., and R. G. Harrison, 2003. Nuclear gene genealogies reveal historical, demographic and selective factors associated with speciation in field crickets. Genetics 163: 1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou, M. L., J. F. Silvain, V. Daubin, J. L. D. Lage and D. Lachaise, 2001. Divergence between Drosophila santomea and allopatric or sympatric populations of D. yakuba using paralogous amylase genes and migration scenarios along the Cameroon volcanic line. Mol. Ecol. 10: 649–660. [DOI] [PubMed] [Google Scholar]
- Chang, A. S., 2004. Conspecific sperm precedence in sister species of Drosophila with overlapping ranges. Evolution 58: 781–789. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., J. A. Coyne and N. Barton, 1987. The relative rates of evolution of sex chromosomes and autosomes. Am. Nat. 130: 113–146. [Google Scholar]
- Clark, A. G., 1997. Neutral behavior of shared polymorphism. Proc. Natl. Acad. Sci. USA 94: 7730–7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeron, J. M., 1999. K-estimator: calculation of the number of nucleotide substitutions per site and the confidence intervals. Bioinformatics 15: 763–764. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1992. Genetics and speciation. Nature 355: 511–515. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 1989. Patterns of speciation in Drosophila. Evolution 43: 362–381. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 1998. The evolutionary genetics of speciation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353: 287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 2004. Speciation. Sinauer Associates, Sunderland, MA.
- Coyne, J. A., S. Y. Kim, A. S. Chang, D. Lachaise and S. Elwyn, 2002. Sexual isolation between sibling species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution 56: 2424–2434. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., S. Elwyn, S. Y. Kim and A. Llopart, 2004. Genetic studies of two sister species in the Drosophila melanogaster subgroup, D. yakuba and D. santomea. Genet. Res. 84: 11–26. [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T., 1937. Genetics and the Origin of Species. Columbia University Press, New York.
- Dvorak, J., M. C. Luo and Z. L. Yang, 1998. Restriction fragment length polymorphism and divergence in the genomic regions of high and low recombination in self-fertilizing and cross-fertilizing aegilops species. Genetics 148: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier, L., P. E. Smouse and J. M. Quattro, 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, C., J. M. Rubio, L. Serrano, J. Gosalvez and G. M. Hewitt, 1993. One way introgression of a subspecific sex chromosome marker in a hybrid zone. Heredity 71: 119–129. [Google Scholar]
- Hey, J., and R. M. Kliman, 1993. Population genetics and phylogenetics of DNA sequence variation at multiple loci within the Drosophila melanogaster species complex. Mol. Biol. Evol. 10: 804–822. [DOI] [PubMed] [Google Scholar]
- Hey, J., and R. Nielsen, 2004. Multilocus methods for estimating population sizes, migration rates and divergence time, with applications to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics 167: 747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton, H., R. M. Kliman and J. Hey, 1994. Using hitchhiking genes to study adaptation and divergence during speciation within the Drosophila melanogaster species complex. Evolution 48: 1900–1913. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., 2001. Two-locus sampling distributions and their application. Genetics 159: 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., and J. A. Coyne, 2002. Mathematical consequences of the genealogical species concept. Evolution 56: 1557–1565. [DOI] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguade, 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 116: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., D. D. Boos and N. L. Kaplan, 1992. A statistical test for detecting geographic subdivision. Mol. Biol. Evol. 9: 138–151. [DOI] [PubMed] [Google Scholar]
- Kimura, M., 1983. The Neutral Theory of Molecular Evolution. Cambridge University Press, Cambridge, UK.
- Kimura, M., and T. Ohta, 1972. The age of a neutral mutant persisting in a finite population. Genetics 75: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman, R. M., P. Andolfatto, J. A. Coyne, F. Depaulis, M. Kreitman et al., 2000. The population genetics of the origin and divergence of the Drosophila simulans complex species. Genetics 156: 1913–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft, T., T. Sall, I. Magnusson-Rading, N. O. Nilsson and C. Hallden, 1998. Positive correlation between recombination rates and levels of genetic variation in natural populations of sea beet (Beta vulgaris subsp. maritima). Genetics 150: 1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaise, D., M. Harry, M. Solignac, F. Lemeunier, V. Bénassi et al., 2000. Evoutionary novelties in islands: Drosophila santomea a new melanogaster sister species from São Tomé. Proc. R. Soc. Lond. Ser. B 267: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley, C. H., J. MacDonald, N. Miyashita and M. Aguade, 1993. Lack of correlation between interspecific divergence and intraspecific polymorphism at the suppressor of forked region in Drosophila melanogaster and Drosophila simulans. Proc. Natl. Acad. Sci. USA 90: 1800–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeunier, F., and M. Ashburner, 1976. Relationship within the melanogaster subgroup of the genus Drosophila (Sophophora). II. Phylogenetic relationships between six species based upon polytene chromosome banding sequences. Proc. R. Soc. Lond. Ser. B 193: 275–294. [DOI] [PubMed] [Google Scholar]
- Llopart, A., S. Elwyn, D. Lachaise and J. A. Coyne, 2002. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 56: 2262–2277. [DOI] [PubMed] [Google Scholar]
- Machado, C. A., and J. Hey, 2003. The causes of phylogenetic conflict in a classic Drosophila species group. Proc. R. Soc. Lond. Ser. B Biol. Sci. 270: 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, C. A., R. M. Kliman, J. A. Markert and J. Hey, 2002. Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol. Biol. Evol. 19: 472–488. [DOI] [PubMed] [Google Scholar]
- Mallet, J., 1995. A species definition for the modern synthesis. Trends Ecol. Evol. 10: 294–299. [DOI] [PubMed] [Google Scholar]
- Mayr, E., 1942. Systematics and the Origin of Species. Columbia University Press, New York.
- Monnerot, M., M. Solignac and D. R. Wolstenholme, 1990. Discrepancy in divergence of the mitochondrial and nuclear genomes of Drosophila teissieri and Drosophila yakuba. J. Mol. Evol. 30: 500–508. [DOI] [PubMed] [Google Scholar]
- Moriyama, E. N., and J. R. Powell, 1997. Synonymous substitution rates in Drosophila: mitochondrial versus nuclear genes. J. Mol. Evol. 45: 378–391. [DOI] [PubMed] [Google Scholar]
- Nachman, M. W., V. L. Bauer, S. L. Crowell and C. F. Aquadro, 1998. DNA variability and recombination rates at X-linked loci in humans. Genetics 150: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, A., and N. H. Barton, 2003. Accumulating postzygotic isolation genes in parapatry: a new twist on chromosomal speciation. Evolution 57: 447–459. [DOI] [PubMed] [Google Scholar]
- Nei, M., and M. W. Feldman, 1972. Identity of genes by descent within and between populations under mutation and migration pressures. Theor. Popul. Biol. 3: 460–465. [DOI] [PubMed] [Google Scholar]
- Nei, M., and T. Gojobori, 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3: 418–426. [DOI] [PubMed] [Google Scholar]
- Noor, M. A. F., K. L. Grams, L. A. Bertucci and J. Reiland, 2001. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. USA 98: 12084–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., and A. J. Betancourt, 2001. Haldane's sieve and adaptation from the standing genetic variation. Genetics 157: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsch, J., 2003. Selective constraints on intron evolution in Drosophila. Genetics 165: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przeworski, M., R. R. Hudson and A. Di Rienzo, 2000. Adjusting the focus on human variation. Trends Genet. 16: 296–302. [DOI] [PubMed] [Google Scholar]
- Ramos-Onsins, S. E., B. E. Stranger, T. Mitchell-Olds and M. Aguadé, 2004. Multilocus analysis of variation and speciation in closely related species Arabidopsis halleri and A. lyrata. Genetics 166: 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, D. M., and L. M. Kann, 1996. Excess amino acid polymorphism in mitochondrial DNA: contrasts among genes from Drosophila, mice, and humans. Mol. Biol. Evol. 13: 735–748. [DOI] [PubMed] [Google Scholar]
- Rand, D. M., M. Dorfsman and L. M. Kann, 1994. Neutral and non-neutral evolution of Drosophila mitochondrial DNA. Genetics 138: 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W., 1989. Analyzing tables of statistical tests. Evolution 43: 223–225. [DOI] [PubMed] [Google Scholar]
- Rieseberg, L. H., C. V. Fossen and A. M. Desrochers, 1995. Hybrid speciation accompanied by genomic reorganization in wild sunflowers. Nature 375: 313–316. [Google Scholar]
- Rozas, J., J. C. Sanchez-DelBarrio, X. Messeguer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496–2497. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and M. Nei, 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 404–425. [DOI] [PubMed] [Google Scholar]
- Shaw, K. L., 2002. Conflict between nuclear and mitochondrial DNA phylogenies of recent species radiation: what mtDNA reveals and conceals about modes of speciation in Hawaiian crickets. Proc. Natl. Acad. Sci. USA 99: 16122–16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. R., 1992. Introgression in fishes: significance for paleontology, cladistics, and evolutionary rates. Syst. Biol. 41: 41–57. [Google Scholar]
- Stephan, W., and C. H. Langley, 1989. Molecular genetic variation in the centromeric region of the X chromosome in three Drosophila ananassae populations. I. Contrasts between the vermilion and forked loci. Genetics 121: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, W., L. Xing, D. A. Kirby and J. M. Braverman, 1998. A test of the background selection hypothesis based on nucleotide data from Drosophila ananassae. Proc. Natl. Acad. Sci. USA 95: 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, M., and P. Donnelly, 2003. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am. J. Hum. Genet. 73: 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, M., N. J. Smith and P. Donnelly, 2001. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima, F., 1989. a DNA polymorphism in a subdivided population: the expected number of segregating sites in the two-subpopulation model. Genetics 123: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima, F., 1989. b Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A., S. C. Tsaur, J. A. Coyne and C.-I Wu, 2001. The nucleotide changes governing pheromonal variation in Drosophila and their evolution. Proc. Natl. Acad. Sci. USA 98: 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano-Shimizu, T., 1999. Local recombination and mutation effects on molecular evolution in Drosophila. Genetics 153: 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., S. Chen, D. L. Hartl and C. C. Laurie, 2003. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the X and autosomes. Genetics 164: 1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, E. B., and J. D. McPhail, 2000. Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus. Proc. R. Soc. Lond Ser. B 267: 2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin and D. G. Higgins, 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting, C. T., S. C. Tsaur and C.-I Wu, 2000. The phylogeny of closely related species as revealed by the genealogy of a speciation gene, Odysseus. Proc. Natl. Acad. Sci. USA 97: 5313–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True, J. R., J. M. Mercer and C. C. Laurie, 1996. Differences in crossover frequency and distribution among three sibling species of Drosophila. Genetics 142: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, P. K., R. D. Sage, J. Warner, A. C. Wilson and E. M. Eicher, 1992. Abrupt cline for sex chromosomes in a hybrid zone between two species of mice. Evolution 46: 1146–1163. [DOI] [PubMed] [Google Scholar]
- Turelli, M., and A. A. Hoffmann, 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeley, J., and J. Hey, 1997. Estimating ancestral population parameters. Genetics 145: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. L., J. Wakeley and J. Hey, 1997. Gene flow and natural selection in the origin of Drosophila pseudoobscura and close relatives. Genetics 147: 1091–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. L., A. Stec, J. Hey, L. Lukens and J. Doebley, 1999. The limits of selection during maize domestication. Nature 398: 236–239. [DOI] [PubMed] [Google Scholar]
- Watterson, G. A., 1975. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 7: 256–276. [DOI] [PubMed] [Google Scholar]
- Wu, C.-I, 2001. The genic view of the process of speciation. J. Evol. Biol. 14: 851–865. [Google Scholar]
- Zabalou, S., S. Charlat, A. Nirgianaki, D. Lachaise, H. Mercot et al., 2004. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics 167: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurovcova, M., and W. F. Eanes, 1999. Lack of nucleotide polymorphism in the Y-linked sperm flagellar dynein gene Dhc-Yh3 of Drosophila melanogaster and D. simulans. Genetics 153: 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]