Abstract
The [URE3] prion of Saccharomyces cerevisiae is a self-propagating inactive form of the nitrogen catabolism regulator Ure2p. To determine whether the [URE3] prion is conserved in S. cerevisiae-related yeast species, we have developed genetic tools allowing the detection of [URE3] in Saccharomyces paradoxus and Saccharomyces uvarum. We found that [URE3] is conserved in S. uvarum. In contrast, [URE3] was not detected in S. paradoxus. The inability of S. paradoxus Ure2p to switch to a prion isoform results from the primary sequence of the protein and not from the lack of cellular cofactors as heterologous Ure2p can propagate [URE3] in this species. Our data therefore demonstrate that [URE3] is conserved only in a subset of Saccharomyces species. Implications of our finding on the physiological and evolutionary meaning of the yeast [URE3] prion are discussed.
PRION is a commonly accepted term to describe the “infectious,” conformationally altered form of an unusual class of proteins found in both mammals and fungi. They were originally implicated in a group of fatal neurodegenerative diseases in mammals, the transmissible spongiform encephalopathies, in which PrPSc, the prion form of the normal protein PrPC, acts as an infectious agent (for reviews see Prusiner et al. 1998; Collinge 2001). In 1982, Prusiner proposed that PrPSc propagates by converting PrPC into PrPSc by an autocatalytic process. Nevertheless, prions are not solely disease-causing agents. Indeed, more recently, it was shown that prions act as novel epigenetic determinants allowing adaptation of cells under certain conditions (True and Lindquist 2000; True et al. 2004). In the case of the [Het-s] prion of the fungus Podospora anserina, the prion form of the protein is the active form in a cell-cell recognition phenomenon (Coustou et al. 1997).
In Saccharomyces cerevisiae, two nonchromosomal elements, [URE3] and [PSI+], discovered a few decades ago (Cox 1965; Aigle and Lacroute 1975), were identified as the prion forms of Ure2p and Sup35p, respectively (Wickner 1994). Ure2p acts as a negative regulator in nitrogen catabolism repression (NCR). In the presence of a good nitrogen source, Ure2p binds the Gln3p transcriptional activator. In turn, this prevents the transcription of a number of genes involved in nitrogen catabolism, including the DAL5 gene that encodes the allantoate permease. [URE3] proved to be an inactive form of Ure2p (Wickner 1994). Consequently, because of a lack of functional Ure2p, [URE3] and ure2 cells can take up poor nitrogen sources even in the presence of good nitrogen sources in the medium (for review see Cooper 2002). Sup35p is involved in translation termination. In [PSI+] cells, termination efficiency is strongly reduced, conferring suppression of nonsense mutations (for reviews see Uptain and Lindquist 2002; Tuite and Koloteva-Levin 2004). Both [URE3] and [PSI+] are dominant in haploid crosses, display a non-Mendelian segregation in meiosis, and are efficiently eliminated (cured) on a medium containing 5 mm guanidine hydrochloride (GuHCl) (for review see Uptain and Lindquist 2002). Ure2p and Sup35p do not share any sequence similarities but in their N-terminal portion both contain stretches of asparagine and glutamine residues, termed the prion forming domain (PFD). The PFD is essential for prion appearance, maintenance, and propagation and is distinct from the functional domain of each protein (Ter-Avanesyan et al. 1994; Masison and Wickner 1995; Derkatch et al. 1996; Masison et al. 1997). In vitro studies showed that these PFDs are responsible for the formation of Ure2p and Sup35p amyloid aggregates, which are thought to be related to the prion-replicating species (Glover et al. 1997; King et al. 1997; Taylor et al. 1999; Thual et al. 1999; King and Diaz-Avalos 2004; Tanaka et al. 2004). Recently, the yeast prion world has become more populated. Rnq1p and New1p, two Asn/Gln-rich-domain-containing proteins, were identified as prions in S. cerevisiae (Santoso et al. 2000; Sondheimer and Lindquist 2000). In silico analyses identified 107 more polypeptides encoded by the S. cerevisiae genome that also contain a Asn/Gln-rich domain, indicating that additional prions might exist in this species (Michelitsch and Weissman 2000).
The physiological relevance of prions in yeast is still an open question. In the case of [PSI+], genetic studies have shown that the prion may allow cells to thrive in certain fluctuating environments (Eaglestone et al. 1999; True and Lindquist 2000). In an attempt to analyze the potential adaptative role of [PSI+], the conservation of [PSI+] has been studied (Chernoff et al. 2000; Kushnirov et al. 2000; Santoso et al. 2000; Jensen et al. 2001; Nakayashiki et al. 2001; Resende et al. 2002, 2003). PFD of full-length genes of SUP35 orthologs from distantly related yeast species have been cloned and their prion properties analyzed in S. cerevisiae (Chernoff et al. 2000; Kushnirov et al. 2000; Santoso et al. 2000; Zadorskii et al. 2000; Nakayashiki et al. 2001; Resende et al. 2002). These studies have shown that all the Sup35p orthologs tested can behave as [PSI+] in S. cerevisiae. While the ability to form [PSI+] seems to be well conserved throughout evolution, the same kind of studies on Ure2p indicate that [URE3] is less conserved. Indeed, several Ure2p orthologs from other yeast species do not behave as prions in S. cerevisiae (Edskes and Wickner 2002; Baudin-Baillieu et al. 2003). However, it is unclear whether the lack of prion behavior of the Ure2p orthologs results from the intrinsic inability of these proteins to adopt the prion isoform or from the lack in S. cerevisiae of species-specific cellular cofactors necessary for prion formation. With one exception (Nakayashiki et al. 2001), all previous studies on the evolutionary biology of yeast prions have been carried out through heterologous expression in S. cerevisiae. These studies thus do not address maintenance or loss of the prion properties of prion protein orthologs in their genuine cellular context. In our study, rather than analyzing heterologous expression of Sup35p or Ure2p orthologs in S. cerevisiae, we chose to directly determine whether [URE3] could exist in non-cerevisiae species.
To gain insight into the conservation of [URE3], we have developed genetic tools to monitor the appearance of [URE3] properties in S. uvarum and S. paradoxus, two yeast species closely related to S. cerevisiae. We first show that the nitrogen regulation function of the tested Ure2p orthologs has been conserved. Then we demonstrate that Ure2p of S. uvarum can behave as a prion in S. uvarum, whereas Ure2p of S. paradoxus cannot behave as a prion in S. paradoxus. Finally, we show that S. cerevisiae Ure2p can adopt a prion isoform in both S. uvarum and S. paradoxus. Our results clearly indicate that the lack of prion properties of Ure2p in S. paradoxus is an intrinsic property of the primary sequence of Ure2p and not due to the lack of species-specific cellular factors. This fact further reveals that [URE3] is not conserved throughout evolution in the Saccharomyces genus, in spite of URE2 ortholog conservation.
MATERIALS AND METHODS
Nomenclature:
To avoid confusion, we used Sc (S. cerevisiae), Sp (S. paradoxus), Su (S. uvarum), and Kl (K. lactis) in subscript to specify the gene origin. For prion nomenclature, [URE3] is used to name the prion status and [ure0] to name the wild-type status of Ure2p.
Plasmids construction:
Table 1 presents the characteristics of all the plasmids used in this study. All the plasmids described below were obtained using the gap repair method (Orr-Weaver and Szostak 1983). Details of the constructions are available upon request. Cloning procedures of the URE2 open reading frames (ORFs) or of the URE2 PFD of the various yeast species into pYeHFn2L were described previously (Baudin-Baillieu et al. 2003). Monocopy plasmids bearing URE2 ORFs were constructed from these plasmids by replacing the 2μ origin with an ARS-CEN origin obtained from pYeHFc1L (Cullin and Minvielle 1994). The LEU2 cassette of pYe2L-URE2ΔC (Baudin-Baillieu et al. 2003) was replaced by the URA3 cassette taken from pYeHFn2U (Cullin and Minvielle 1994) to obtain pYe2U-URE2ΔC.
TABLE 1.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pYeHFn2L | 2μ LEU2 | Cullin and Minvielle (1994) |
| pYe2L-URE2ScΔC | 2μ LEU2 PGAL10 URE2ScΔC | Baudin-Baillieu et al. (2003) |
| pYe2L-URE2SpΔC | 2μ LEU2 PGAL10 URE2SpΔC | Baudin-Baillieu et al. (2003) |
| pYe2L-URE2SuΔC | 2μ LEU2 PGAL10 URE2SuΔC | Baudin-Baillieu et al. (2003) |
| pYe2L-URE2KlΔC | 2μ LEU2 PGAL10 URE2KlΔC | Baudin-Baillieu et al. (2003) |
| pYeHFn2U | 2μ URA3 | Cullin and Minvielle (1994) |
| pYe2U-URE2ScΔC | 2μ URA3 PGAL10 URE2ScΔC | This study |
| pYe2U-URE2SpΔC | 2μ URA3 PGAL10 URE2SpΔC | This study |
| pYe2U-URE2SuΔC | 2μ URA3 PGAL10 URE2SuΔC | This study |
| pYe2U-URE2KlΔC | 2μ URA3 PGAL10 URE2KlΔC | This study |
| pYe1L-URE2Sc | CEN LEU2 PGAL10 URE2Sc | This study |
| pYe1L-URE2Sp | CEN LEU2 PGAL10 URE2Sp | This study |
| pYe1L-URE2Su | CEN LEU2 PGAL10 URE2Su | This study |
| pYe1L-URE2Kl | CEN LEU2 PGAL10 URE2Kl | This study |
| pYe2L-URE2Sp | 2μ LEU2 PGAL10 URE2Sp | Baudin-Baillieu et al. (2003) |
| pH660 | 2μ LEU2 PGAL1 URE2Sp | Edskes and Wickner (2002) |
To get the pYe2L-DAL5Su plasmid, the DAL5Su ORF, its promoter (319 bp upstream), and its terminator (317 bp downstream) were PCR amplified from the Su1a strain and cloned into pYeHFn2L between the BamHI and Bsu36I sites. From this plasmid, to get the pYe2L-pDAL5Su∷ADE2Sc plasmid, the DAL5Su ORF was replaced by the one of ADE2Sc, PCR amplified from the pYeHFn2A plasmid. To get the pYe2L-URE2Su plasmid, the URE2Su gene was PCR amplified from strain Su1a and cloned into pYeHFn2L between the BamHI and Bsu36I sites. To get the pYe2L-ure2Su∷URA3Sc, a part of URE2Su was replaced by the URA3Sc gene, PCR amplified from the pYeHFn2U plasmid. To get the pYe2L-HOSp plasmid, the HOSp gene was PCR amplified from strain Sp4707-22D and cloned into the pYeHFn2L plasmid between the BamHI and Bsu36I sites. From this plasmid, to get the pYe2L-hoSp∷KanMX4 plasmid, the HO ORF was replaced by the KanMX4 cassette, PCR amplified from the pFA-6A plasmid (Wach et al. 1994). To get the pYe2L-DAL5Sp, the DAL5Sp ORF, its promoter (389 bp upstream), and its terminator (447 bp downstream) were PCR amplified from strain Sp4707-22D and cloned into pYeHFn2L between the BamHI and Bsu36I sites. With this plasmid, the ORF of DAL5Sp was replaced by the ORF of ADE2Sc, PCR amplified from the pYeHFn2A plasmid, resulting in the pYe2L-pDAL5Sp∷ADE2Sc plasmid. To create the pYe2L-URE2Sp plasmid, the URE2Sp gene was PCR amplified from strain Sp4707-22D and cloned into the pYeHFn2L between the BamHI and Bsu36I sites. From this plasmid, to make the pYe2L-ure2Sp∷LYS1Sc plasmid, a part of URE2Sp was replaced by the LYS1 gene, PCR amplified from genomic DNA of S. cerevisiae. For each plasmid, a test was performed in corresponding strains to check the functionality of the clones' ORFs. All sequences were obtained from the Génolevure project (Souciet et al. 2000), the genomic sequence projects (Cliften et al. 2003; Kellis et al. 2003), or from our data. The pH660 plasmid was kindly provided by Reed Wickner (Edskes and Wickner 2002).
Strain construction:
All strains used in this study are listed in Table 2. Details of the constructions are available upon request. S. uvarum Su[ure0] was constructed from strain Su5-1A (Talarek et al. 2004) by transformation with a PCR product containing the dal5Su∷ADE2Sc cassette amplified from pYe2L-pDAL5Su∷ADE2Sc (the correct notation should be dal5∷PDAL5 ADE2; for the sake of simplicity we noted the construction as pDAL5∷ADE2). Integrative transformants were selected on minimal medium without adenine and with proline as poor nitrogen source. The S. uvarum strain Su(Δure2) was constructed by transformation of the S. uvarum strain Su[ure0] with a PCR product containing the ure2Su∷URA3Sc cassette amplified from pYe2L-ure2Su∷URA3Sc. Integrative transformants were selected on SD medium without adenine-containing ammonia as a good nitrogen source. Sp4707-22D, Sp4795-3B′/D, and Sp2B12D S. paradoxus original strains were used. From these strains, heterothallic strains were obtained by transformation with a PCR product containing the hoSp∷KanMX4 cassette from the plasmid pYe2L-hoSp∷KanMX4. Integrative transformants were selected on rich medium containing 200 mg/liter G418 (Sigma, St. Louis). S. paradoxus strain Sp[ure0] was constructed from S. paradoxus ade2 strain by transformation with a PCR product containing the pDAL5Sp∷ADE2Sc cassette, obtained from the plasmid pYe2L-pDAL5Sp∷ADE2Sc. Integrative transformants were selected on minimal medium without adenine and with a poor nitrogen source. The S. paradoxus Sp(Δure2) strain was constructed by transformation of S. paradoxus strain Sp12B with a PCR product containing the ure2Sp∷LYS1Sc, obtained from pYe2L-ure2Sp∷LYS1Sc. Integrative transformants were selected on SD medium without adenine containing a good nitrogen source. In each case, to confirm the disruption, transformants were analyzed by PCR. All transformants were also crossed with a strain of opposite mating type and the resulting diploids were sporulated and dissected to obtain strains with opposite mating type.
TABLE 2.
Strains used in this study
| Strain | Species | Genotype | Reference |
|---|---|---|---|
| Sc[ure0] | S. cerevisiae | MATahis3-11,15 leu2-3,112 ade2-1 trp1-1 ura2∷HIS3 pDAL5∷ADE2 | Bach et al. (2003) |
| Sc[URE3] | S. cerevisiae | MATahis3-11,15 leu2-3,112 ade2-1 trp1-1 ura2∷HIS3 pDAL5∷ADE2 [URE3] | Bach et al. (2003) |
| Su5-1A | S. uvarum | MATa, ura3-1 leu2∷URA3 ade2∷URA3 | Talarek et al. (2004) |
| Su[ure0] | S. uvarum | MATα ura3-1 leu2∷URA3 ade2∷URA3 pDAL5∷ADE2 | This study |
| Su[URE3]S | S. uvarum | MATα ura3-1 leu2∷URA3 ade2∷URA3 pDAL5∷ADE2 [URE3]Sa | This study |
| Su[URE3]I | S. uvarum | MATα ura3-1 leu2∷URA3 ade2∷URA3 pDAL5∷ADE2 [URE3]Ib | This study |
| Su(Δure2) | S. uvarum | MATa, ura3-1, leu2∷URA3, ade2∷URA3, ure2∷URA3, pDAL5∷ADE2 | This study |
| Sp4707-22D | S. paradoxus | MATaaahis4 ura3 ade1 leu2 | Hawthorne and Philippsen (1994) |
| Sp4795-3B′/D | S. paradoxus | MATααα met1 ura1 ade2 aro7 | Hawthorne and Philippsen (1994) |
| Sp2B12D | S. paradoxus | a/α trp5x/trp5y ade2/+ ade5,7/+ +/ade1 leu1x/ leu1y his4/+ lys1/+ are4/+ met13x/met13y | Herbert et al. (1988) |
| Sp12B | S. paradoxus | MATaade2 leu2 his4 lys1 pDAL5∷ADE2 | This study |
| Sp[ure0] | S. paradoxus | MATaho∷KanMX4 his4 leu2 ade2 ura3 pDAL5∷ADE2 | This study |
| Sp(Δure2) | S. paradoxus | MATaho∷KanMX4 his4 leu2 ade2 ura3 lys1 ure2∷ LYS1 pDAL5∷ADE2 | This study |
| CC30 | S. cerevisiae | MATahis3-11,15 leu2-3,112 ade2-1 trp1-1 ura2∷HIS3 | Fernandez-Bellot et al. (2000) |
[URE3]S, the S stands for spontaneous [URE3].
[URE3]I, the I stands for induced [URE3].
Medium and microbiological methods:
Yeast cells were grown at 30° according to methods previously described for S. cerevisiae (Sherman 1991). YPDA was the YPD rich medium supplemented with 20 mg/liter of adenine. Synthetic dextrose (SD: 2% dextrose, 0.67% yeast nitrogen base with ammonia) was used as selective medium. The color phenotype was assayed on YPD4 medium [1% peptone, 1% yeast extract (Fisher), 4% dextrose]. Color phenotypes (white to dark red) were checked after 5–7 days at 30° and 2 days at 4°. The ureidosuccinic acid (USA) uptake phenotype in ura2 strains was tested on SD medium to which the required amino acids and bases (except uracil) were added as well as 15 μg/ml USA (pH 6.7). Induction of the galactose promoter was performed on appropriate synthetic glucose-free medium containing 2% galactose (SG) and, if necessary, 2% raffinose. All mating, sporulation, and dissection procedures were carried out according to standard protocols (Sherman and Hicks 1991). For S. uvarum and S. paradoxus species, procedures were performed as previously described (Talarek et al. 2004). Asci dissections were performed by micromanipulation (Singer Instrument MSM). Curing [URE3] by GuHCl was performed in liquid or solid medium. In liquid medium, cells were grown for 20 generations in the presence of 2.5 mm or 5 mm GuHCl in YPD at 30°. The resulting population was then tested for [Ade] phenotype. In solid medium, a drop of [URE3] cells was put on YPD4 medium containing 2.5 mm or 5 mm GuHCl. The white/red phenotype was checked after 5–7 days at 30°.
Ure2p solubility assay by subcellular fractionation:
Yeast [URE3] cells were grown in SD medium without adenine (in SD medium containing adenine for the [ure0] strain) to exponential phase and then diluted into YPDA medium and grown for 5 more hours. Total protein extract and fraction preparation were done as described previously (Ripaud et al. 2003). Urea was added to each fraction to a final concentration of 8 m. Prior to loading, the samples were boiled for 5 min. Equal quantities of each sample were analyzed by Western blot (12% SDS-PAGE, tricine buffer). Proteins were transferred onto nitrocellulose membranes, revealed with the ECL+ reagent (Pierce, Rockford, IL), and recorded with the VersaDoc Imaging System (Bio-Rad, Hercules, CA). Ure2p was quantified with Quantity One software (Bio-Rad). Polyclonal antibodies raised against Ure2p were affinity purified and diluted 1/3000 (Fernandez-Bellot et al. 2000).
RESULTS
Ure2p function is conserved among species:
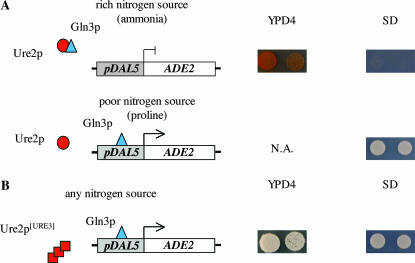
To analyze the conservation of [URE3] in the Saccharomyces genus, we searched for [URE3] cells in S. paradoxus and S. uvarum. For this purpose, we developed a reporter system allowing an easy detection of [URE3] in these species. We adapted the reporter system previously described by Schlumpberger et al. (2001) for S. cerevisiae and used this as a secondary screen for the identification of antiprion molecules (Bach et al. 2003). In this system the ADE2 open reading frame is under the control of the DAL5 promoter and inserted at the DAL5 locus. ADE2 transcription is thus under the control of the Gln3p transcriptional activator, which is inhibited by Ure2p in the presence of a good nitrogen source (Figure 1) (for review see Cooper 2002). Therefore, on rich and minimal media containing ammonia (a good nitrogen source), the [ure0] cells are red and [Ade−], whereas the [URE3] cells are white and [Ade+]. Thus, the use of this reporter system allows discrimination between [URE3] and [ure0] cells simply by checking their color and their auxotrophy for adenine on rich and minimal media, respectively.
Figure 1.
Reporter system used to detect [URE3] in S. cerevisiae, S. paradoxus, and S. uvarum. (A) [ure0]: Ure2p in normal state. In an ade2 strain on rich or minimal medium containing a good nitrogen source (such as ammonia), Ure2p binds Gln3p and prevents the transcription of ADE2 from the DAL5 promoter. On rich medium (YPD4) where adenine is limiting, colonies are red due to the lack of Ade2p activity. They cannot grow on glucose minimal medium without adenine (SD). On glucose minimal medium (SD) containing proline, a poor nitrogen source, Ure2p does not bind Gln3p. Gln3p can thus activate the transcription of ADE2 from the DAL5 promoter. Colonies are therefore [Ade+] on proline medium. (B) [URE3]: Ure2p in prion state. In the [URE3] state, Ure2p is in an inactive form, and Gln3p can activate the transcription of ADE2 from the DAL5 promoter whatever the nitrogen source. Colonies are white on rich medium (YPD4) and [Ade+] on SD medium. Due to the lack of Ure2p, an ure2 mutant displays the same phenotype as that of an [URE3] strain. NA, not appropriate.
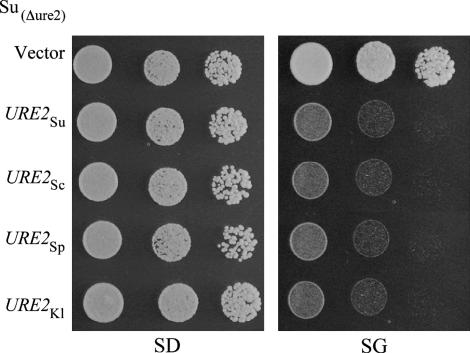
All URE2 orthologs studied so far share much more similarity in their C-terminal functional domain than in their N-terminal PFD. They retain their Ure2p function when expressed in S. cerevisiae (Edskes and Wickner 2002; Baudin-Baillieu et al. 2003). Before testing the prion properties of Ure2p(Sc,Sp,Su,Kl) orthologs, we determined whether they could complement an URE2 deletion in S. uvarum and S. paradoxus. To this purpose we transformed our reporter Δure2 strains with plasmids expressing the Ure2p orthologs from a galactose-inducible promoter. The S. uvarum Su(Δure2) strain was [Ade+] on glucose minimal medium (Figure 2, SD). On galactose minimal medium, when Ure2pSu was expressed, the strains became [Ade−]. The same result was observed with the other Ure2p orthologs (Figure 2, SG), indicating that all the orthologs complemented the URE2 gene deletion in S. uvarum. We performed the same assay with the S. paradoxus Sp(Δure2) strain and obtained the same results (data not shown). Thus the Ure2p function in nitrogen catabolism repression is conserved among the studied orthologs and this allowed us to study the conservation of their prion properties.
Figure 2.
Complementation of ure2 by Ure2p orthologs. The S. uvarum Su (Δure2) strain was transformed by plasmids carrying URE2 orthologs under the control of a galactose-inducible promoter control (pYe2L-URE2). All the strains grew on dextrose minimal medium (SD) without adenine, because the absence of the URE2 function allows the transcription of the reporter system. On galactose minimal medium without adenine (SG), strains did not grow, indicating that Ure2p was expressed and prevented the transcription of the ADE2 gene.
[URE3] appears spontaneously in S. uvarum but not in S. paradoxus:
To determine whether or not [URE3] exists in S. paradoxus and S. uvarum, we searched for spontaneous [URE3] in S. uvarum and S. paradoxus. [ure0] S. cerevisiae, S. paradoxus, and S. uvarum strains (Sc[ure0], Sp[ure0] and Su[ure0], respectively) were grown on SD medium without adenine. Several [Ade+] clones were obtained for each species (Table 3). Then the [URE3] status of 200 [Ade+] clones was tested using three criteria. To be scored as [URE3], the [Ade+] clones had to display the following characteristics. First, adenine prototrophy should be cured after GuHCl treatment; second it should be dominant in a cross with an ade2 haploid; and third, it should display a non-Mendelian segregation in tetrads obtained after sporulation of the resulting diploid. As shown in Table 3, for S. cerevisiae the spontaneous frequency of appearance of [URE3] was 6.8 × 10−6, a frequency comparable to those obtained in previous studies (Wickner 1994). For S. uvarum the spontaneous frequency of appearance of [URE3] was 4.2 × 10−5, slightly higher than that observed for S. cerevisiae. This result indicates that Ure2pSu can spontaneously adopt a prion isoform in S. uvarum. To our knowledge, this is the first report of a spontaneous emergence of a yeast prion in a yeast species other than S. cerevisiae. For S. paradoxus, none of the tested [Ade+] clones were [URE3]. Thus, in S. paradoxus, Ure2pSp did not spontaneously adopt a prion state at a frequency detectable with our reporter system. This strongly suggests that [URE3] is not conserved in all Saccharomyces species.
TABLE 3.
Spontaneous appearance of [URE3] in S. cerevisiae, S. paradoxus, and S. uvarum
| Species | [Ade+] per 107 cells | [URE3] frequency |
|---|---|---|
| S. cerevisiae | 710 | 6.8 × 10−6 |
| S. paradoxus | 220 | <10−7 |
| S. uvarum | 830 | 4.2 × 10−5 |
After overnight growth in YPD rich medium, 107 cells were plated onto SD medium without adenine. After 8 days of growth, the number of [Ade+] were counted. The [URE3] status of 200 [Ade+] clones was then monitored as described in the results (cure, dominance, segregation) except for S. paradoxus where all the [Ade+] clones were analyzed. All the [Ade+] clones that were cured by guanidine hydrochloride displayed a non-Mendelian segregation.
Ure2p PFDs can induce [URE3] in S. uvarum but not in S. paradoxus:
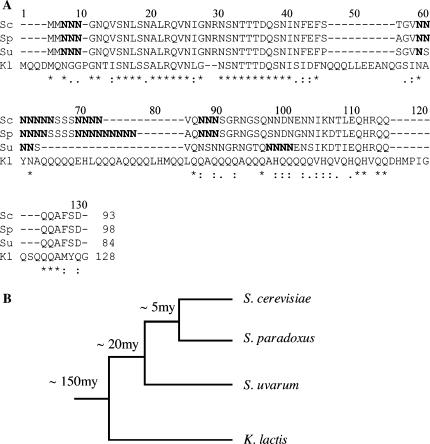
Ure2pSc consists of an N terminus PFD (residues 1–90) and a C-terminal domain (residues 91–354) that is required for activity and resembles glutathione S-transferases in sequence and structure and that has been proved to have glutathione peroxydase activity (Coschigano and Magasanik 1991; Bousset et al. 2001; Umland et al. 2001; Bai et al. 2004). In S. cerevisiae, overproduction of the Ure2pSc PFD strongly increases the frequency of [URE3] appearance (Masison and Wickner 1995; Maddelein and Wickner 1999). We asked whether the overexpression of PFDs from Ure2p orthologs, as they were previously described (Figure 3A and Baudin-Baillieu et al. 2003), could increase [URE3] frequency in S. uvarum and promote [URE3] appearance in S. paradoxus. We transformed strains with plasmids expressing PFDs from a galactose-inducible promoter and analyzed the [URE3] phenotype of the resulting [Ade+] clones obtained upon overexpression of PFD. In the S. uvarum Su[ure0] strain, overexpression of the PFDSu induced a threefold increase in the frequency of [URE3] appearance (Table 4A). Heterologous PFDSc and PFDSp were more effective than PFDSu, inducing a 13-fold and a 25-fold increase in this frequency, respectively. By contrast, overexpression of the PFDKl had no effect on the frequency of [URE3] appearance (Table 4A). These results parallel the one obtained in S. cerevisiae (Baudin-Baillieu et al. 2003). Thus, the PFDs capable of inducing [URE3] in S. cerevisiae were also able to induce [URE3] in S. uvarum and, conversely, the PFD (PFDKl) that failed to induce [URE3] in S. cerevisiae is also inactive in [URE3] in S. uvarum induction (Baudin-Baillieu et al. 2003). The different PFDs were also overexpressed in the S. paradoxus Sp[ure0] strain and [Ade+] clones were recovered. The frequency of [Ade+] clones obtained is seemingly the same with an empty vector or with any overexpressed PFD (Table 4B). Among 200 [Ade+] clones tested, none were curable by GuHCl treatment and all displayed a Mendelian segregation (Table 4B). Thus we concluded that no [URE3] clones were obtained, indicating that Ure2pSp could not adopt a prion isoform despite a strong overexpression of PFDs. It thus appears that Ure2pSp cannot adopt a prion isoform in S. paradoxus either spontaneously or upon induction with PFDs.
Figure 3.
Evolutionary analysis of Ure2p PFDs. (A) Multiple alignments of the Ure2p orthologs PFDs. The sequences are from a previous study (Baudin-Baillieu et al. 2003). Identities are indicated with an asterisk, strong and weak similarities with two and one point, respectively. Main Asn stretches are in boldface type. Sequences were aligned using the ClustalW algorithm (Higgins et al. 1996). (B) Phylogenetic relationships of yeast based on DNA sequences (Cliften et al. 2003; Kellis et al. 2003). The evolutionary distance was expressed in millions of years (my).
TABLE 4.
[URE3] induction by overexpression of PFD in S. uvarum Su[ure0] and S. paradoxus Sp[ure0] strains
| Plasmid | [URE3] frequency | Induction fold above the spontaneous frequency |
|---|---|---|
| A. In S. uvarum | ||
| Vector | 2.1 × 10−6 | |
| PFDSc | 2.7 × 10−5 | 13 |
| PFDSp | 5.2 × 10−5 | 25 |
| PFDSu | 6.0 × 10−6 | 3 |
| PFDKl | 1.9 × 10−6 | 1 |
| Plasmid | [Ade+] frequency | [URE3] frequency |
| B. In S. paradoxus | ||
| Vector | 3.11 × 10−5 | |
| PFDSc | 2.40 × 10−5 | <10−7 |
| PFDSp | 4.05 × 10−5 | <10−7 |
| PFDSu | 1.55 × 10−5 | <10−7 |
| PFDKl | 1.44 × 10−5 | <10−7 |
Strains were transformed with plasmids allowing the overexpression of the different PFD(Sc,Sp,Su,Kl) from a galactose-inducible promoter (pYe2L-URE2(Sc,Sp,Su,Kl)ΔC). Some clones of transformed strains were grown on raffinose/galactose minimal medium for 72 hr to induce overexpression. Then cells were plated onto glucose SD medium. After 5 days of growth, [ADE+] clones were collected and their [URE3] state was then monitored as described in the results. The [URE3] state of 200 [Ade+] clones was then monitored as described in the results. It should be noted that upon overexpression with PFDSc, PFDSp, and PFDSu, in S. uvarum all the [Ade+] clones obtained were [URE3].
Characterization of two distinct [URE3]Su strains in S. uvarum:
Another aspect of prion biology is the existence of different prion strains that were first identified in mammals (Bruce et al. 1991). In S. cerevisiae, different [URE3] (Schlumpberger et al. 2001) and [PSI+] strains (Uptain et al. 2001; Bradley et al. 2002; King and Diaz-Avalos 2004; Tanaka et al. 2004) have also been distinguished, depending on the strength of the phenotype. Genetic studies have shown that prion strains differ by their mitotic stability. In the case of Sup35p, the different [PSI+] strains are characterized by differing amounts of Sup35p in the aggregated form and by the existence of different amyloid conformations (Uptain et al. 2001; Bradley et al. 2002; King and Diaz-Avalos 2004; Tanaka et al. 2004).
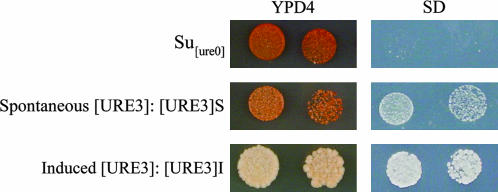
In S. uvarum, [URE3]Su clones were obtained either spontaneously or upon overexpression of PFDs. All spontaneous S. uvarum [URE3] clones gave rise to light-red colonies on rich medium and grew poorly on SD medium (Figure 4; see [URE3]S; S for spontaneous). Upon PFD overexpression, all [URE3] clones gave rise to white colonies on rich medium and grew as well on SD medium (Figure 4, [URE3]I, I signifying induced) as the wild type does (data not shown). To further differentiate between these two [URE3]Su strains, we analyzed the mitotic and meiotic stability, dominance, and Ure2p solubility for each strain (Table 5).
Figure 4.
Comparison of color phenotype and adenine prototrophy in S. uvarum Su[ure0] colonies and in two kinds of S. uvarum [URE3] strains, Su[URE3]S and Su[URE3]I, obtained spontaneously and after PFD overexpression, respectively. Cells were grown on YPD4 and on SD medium at 30° for 5–7 days. The S and I indicate spontaneous and induced [URE3], respectively.
TABLE 5.
Mitotic and meiotic stability and dominance of Su[URE3]S and Su[URE3]I
| Mitotic stability (%)
|
[URE3] diploid (%)
|
Meiotic stability (%)
|
|||||
|---|---|---|---|---|---|---|---|
| Strain | 4∷0 | 3∷1 | 2∷2 | 1∷3 | 0∷4 | ||
| Su[URE3]S | 20 | 30 | 32 | 29 | 29 | 7 | 3 |
| Su[URE3]I | 92 | 90 | 90 | 7 | 3 | 0 | 0 |
Mitotic stability was determined by counting the ratio of [Ade+] clones after 20 generations in YPDA rich medium (2000 cells were counted for each strain). Dominance was determined by monitoring [Ade+] diploids obtained after crosses between wild-type and [URE3] strains (30 diploids were counted for each strain). After sporulation and dissection of the resulting [Ade+] diploids, meiotic stability was determined by analyzing the [URE3] segregation ([URE3]∷[ure0]) (120 tetrads were dissected for each strain).
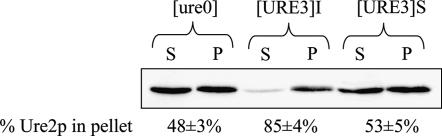
Growth of both strains in YPDA during 20 generations showed that [URE3]I is more stable mitotically than [URE3]S. We then crossed the S. uvarum Su[ure0] strain with both [URE3] strains and analyzed the [URE3] status of the resulting diploids. This result indicated that [URE3]I is more invasive than [URE3]S. The phenotype of the progeny after sporulation of the previously obtained [URE3] diploids revealed that [URE3]I is meiotically more stable than [URE3]S. We then analyzed the solubility of Ure2p by subcellular fractionation (Figure 5). This indicated that more of the Ure2p was found in pellet fraction in the Su[URE3]I strain than in the Su[URE3]S strain. This suggests that overexpression of PFD leads to a strong [URE3] strain and a weak [URE3] strain was obtained spontaneously.
Figure 5.
Ure2p solubility in S. uvarum Su[ure0], Su[URE3]S, and Su[URE3]I strains. Su[ure0] is the [ure0] S. uvarum strain, and the Su[URE3]S strain corresponds to the S. uvarum spontaneous [URE3] (Figure 4, Table 3). The Su[URE3]I strain corresponds to the S. uvarum [URE3] obtained after overexpression of PFD (Figure 4, Table 4). We determined the subcellular distribution of Ure2p and the percentage of Ure2p in the 100,000 × g pellet fraction (% Ure2p in the pellet) as described in materials and methods. S, supernatant fraction; P, pellet fraction. The values were obtained from three independent experiments.
S. paradoxus can propagate [URE3]Sc and [URE3]Su:
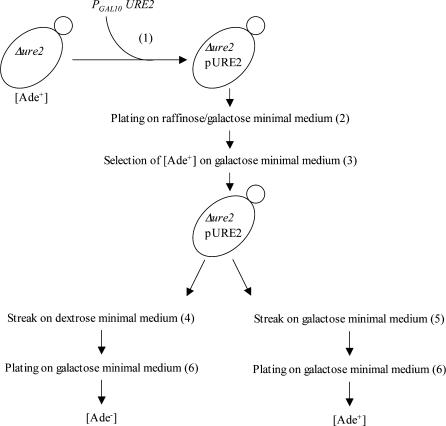
Unlike Ure2pSu, Ure2pSp cannot adopt a prion isoform in S. cerevisiae spontaneously, upon overexpression with PFDs, or in the presence of a preexisting [URE3]Sc (Baudin-Baillieu et al. 2003). We showed above that Ure2pSp cannot adopt a prion isoform in S. paradoxus either spontaneously or upon overexpression of PFD. The lack of [URE3] in S. paradoxus could result from either an intrinsic inability of Ure2pSp to switch to the prion state or the lack of cellular cofactors necessary for prion propagation (for review see Osherovich and Weissman 2002; Uptain and Lindquist 2002). To check if S. paradoxus displays all the necessary cofactors required for [URE3] propagation, we determined whether Ure2pSc and Ure2pSu are able to give rise to [URE3] in this species. To avoid any cross-reaction, the S. paradoxus Sp(Δure2) strain was used. This strain was transformed with plasmids overexpressing either Ure2pSc or Ure2pSu from a galactose-inducible promoter. The method used to determine the [URE3] status is summarized in Figure 6. As the initial Δure2 strain has the same phenotype as potential [URE3] clones, this method is rather complex and is based on the fact that the prion phenotype cannot be maintained in the transient absence of Ure2p expression. [URE3] was obtained when Ure2pSc or Ure2pSu was overexpressed (Table 6). These results indicated that S. paradoxus can harbor a [URE3] phenotype and that all the cellular cofactors required to induce, maintain, and propagate [URE3] are present in this species.
Figure 6.
Induction of [URE3]Sc in S. uvarum Su(Δure2) and in S. paradoxus Sp(Δure2) strains. (1) Cells were transformed with a plasmid, allowing the overexpression of Ure2p (pYe1L-URE2) from a galactose-inducible promoter. (2) Transformed strains were plated onto raffinose/galactose minimal medium supplemented with adenine. After 3 days, these strains were transferred onto galactose minimal medium without adenine to select [Ade+] clones (3). To test the [URE3] status of the [Ade+] clones, clones were replica plated on dextrose minimal medium to switch off the overexpression of Ure2p (4). Indeed, [URE3] cannot be maintained without continuous URE2 expression. Clones remained on galactose (5) and one replica plated on dextrose minimal medium (4) were tested again on galactose minimal medium without adenine (6). Clones were [URE3] when cells remaining on galactose were [Ade+] and cells replica plated on glucose became [Ade−]. The [URE3] status was confirmed by GuHCl treatments.
TABLE 6.
Ure2p of S. cerevisiae and S. uvarum adopt a prion isoform in S. paradoxus
| Ortholog | [URE3] frequency |
|---|---|
| URE2Sc | 5 × 10−6 |
| URE2Sp | 0 |
| URE2Su | 1 × 10−6 |
| URE2Kl | 0 |
The Sp(Δure2) strain was transformed with plasmids expressing Ure2p orthologs. The [URE3] phenotype was tested as described in Figure 6. The [URE3] state of 200 [Ade+] clones was monitored.
To further document prion conversion in a heterologous cellular context, we also tested, in the same way, whether Ure2p(Sc,Sp,Kl) can adopt a prion isoform in S. uvarum. To avoid any cross-reaction, the S. uvarum Su(Δure2) strain was used. This strain was transformed with plasmids overexpressing Ure2p orthologs from a galactose-inducible promoter. In the S. uvarum Su(Δure2) strain, [URE3] cells were obtained by overexpression of Ure2pSc or Ure2pSu (Table 7). This result confirmed that in S. uvarum cellular cofactors required to induce, maintain, and propagate [URE3] were present. No [URE3] clones were obtained when Ure2pSp or Ure2pKl was overexpressed (Table 7), implying that these proteins themselves do not contain sequences necessary to allow prion formation and/or propagation.
TABLE 7.
Prion properties of Ure2p orthologs in S. uvarum
| Ortholog | [URE3] frequency |
|---|---|
| URE2Sc | 1 × 10−5 |
| URE2Sp | 0 |
| URE2Su | 2 × 10−6 |
| URE2Kl | 0 |
The Su(Δure2) strain was transformed with plasmids expressing Ure2p orthologs. The [URE3] phenotype was tested as described in the legend of Figure 6. The [URE3] state of 200 [Ade+] clones was monitored.
Ure2pSp cannot adopt a prion isoform in S. paradoxus even in the presence of a preexisting heterologous [URE3]:
We could hypothesize that Ure2pSp, which failed to spontaneously adopt a prion isoform in S. paradoxus, might be converted into a prion form in the presence of preexisting [URE3]. Therefore we tested whether the Ure2pSp protein could be converted into a prion isoform in the presence of [URE3]Sc in S. paradoxus. We crossed a [URE3]Sc Δure2 S. paradoxus haploid strain (see above) with a S. paradoxus Sp[ure0] strain on galactose minimal medium to maintain Ure2pSc expression. In the diploid strain both Ure2pSc and Ure2pSp are expressed. If Ure2pSp is not converted into [URE3], the diploid should remain wild type for Ure2pSp function. Among the 30 tested diploids, none was [Ade+], showing that Ure2Sp remained in its functional isoform (data not shown). This result indicated that Ure2pSp cannot adopt a prion isoform even in the presence of a preexisting [URE3].
DISCUSSION
One of the most intriguing questions surrounding fungal prions concerns their potential biological role. Prion properties of many orthologs of Ure2p and Sup35p have been studied in S. cerevisiae (Chernoff et al. 2000; Kushnirov et al. 2000; Santoso et al. 2000; Nakayashiki et al. 2001; Edskes and Wickner 2002; Resende et al. 2002; Baudin-Baillieu et al. 2003), but so far only one study has addressed the aggregation of Sup35p in a yeast species other than S. cerevisiae (Nakayashiki et al. 2001). Here we demonstrate that [URE3] can be obtained either spontaneously or upon overexpression of PFD in S. uvarum. Concerning S. paradoxus, two contradictory results have been obtained with Ure2pSp when it has been expressed in S. cerevisiae (Edskes and Wickner 2002; Baudin-Baillieu et al. 2003). More precise analyses of these results (Edskes and Wickner 2002) tend to show that a readthrough phenomenon explains these contradictory results (supplemental data at http://www.genetics.org/supplemental/). Although we cannot formally rule out that [URE3] might exist in an atypical form in S. paradoxus with an extremely low probability of appearance, we have never obtained [URE3]Sp spontaneously, upon overexpression of PFD, or in the presence of preexisting [URE3]. We conclude that typical [URE3] cannot be formed in S. paradoxus. This suggests that [URE3] is not conserved throughout the Saccharomyces genus. Thus the presence of [URE3] is not correlated with the phylogenetic tree since S. paradoxus (which lacks [URE3]) is a more closely related species to S. cerevisiae than is S. uvarum (which harbors [URE3]) (Figure 3B).
Conservation of prion properties is mediated by primary sequence rather than by cellular factors:
Among the Ure2p and Sup35p orthologs tested in S. cerevisiae, some exhibit prion properties whereas others do not. Two hypotheses may be proposed to explain this observation. First, species-specific cellular factors could allow or prevent prion apparition. Second, prion properties could be associated with an intrinsic behavior of each protein. We found that both Ure2Sc and Ure2Su, two orthologs that can behave as prions in their own cellular context, can adopt a prion isoform in all three tested species. Consequently, all species possess the cellular factors necessary for [URE3] propagation. However, in S. paradoxus, Ure2Sp cannot adopt a prion isoform spontaneously, upon overexpression of PFDs, or even in the presence of a preexisting [URE3]Sc. Thus Ure2pSp does not behave as a prion in species that possess a cellular context permissive to prion emergence (this study and Baudin-Baillieu et al. 2003). These data suggest that the conservation of prion properties is mediated by the protein itself rather than by cellular cofactors. Further, the main cellular cofactors allowing prion emergence and propagation appear to be functionally conserved throughout the Saccharomyces genus.
An Asn/Gln-rich domain is not sufficient to confer prion properties:
Several lines of evidence have indicated that the PFD of yeast prion proteins is necessary and sufficient for prion formation (Tuite 2000). In the case of Ure2p orthologs, PFDs have been defined from multiple alignments (Baudin-Baillieu et al. 2003 and Figure 3A). All these PFDs share a high content of Asn/Gln residues. Although the three PFDs that share a high degree of identity are able to induce [URE3] in both S. cerevisiae and S. uvarum, the PFDKl that contains a stretch of glutamines does not retain the prion-inducing properties in these two species. The abundance of Asn/Gln residues in a protein sequence has been used as a criterion to identify new potential prions in S. cerevisiae (Michelitsch and Weissman 2000). In addition, recent data indicate that [URE3] prion formation is driven primarily by the amino acid composition of the PFD, largely independent of its primary sequence (Ross et al. 2004). However, our results indicate that a high content of Asn/Gln does not systematically confer prion-inducing properties when it is attached to its globular domain. Indeed, expressed alone, the PFDSp is able to induce [URE3] in S. cerevisiae and S. uvarum. However, when it is attached to the globular domain, its prion-inducing properties are lost. These data confirm that an Asn/Gln-rich domain is not sufficient to determine whether a protein can behave as a prion and that the prion-inducing properties of an Asn/Gln-rich domain can be very different when the Asn/Gln-rich domain is embedded in the full-length protein. It should be noted that S. cerevisiae and S. paradoxus Ure2p's share the exact same sequence in their globular domains only and that the differences are contained in the PFDs. Several results suggest that the PFD and the C-terminal domain of Ure2pSc interact functionally with each other, leading to an inhibitory effect on the acquisition of the prion state (Fernandez-Bellot et al. 1999, 2000; Maddelein and Wickner 1999). However, it has been suggested that there are no physical interactions between the two domains of Ure2pSc (Pierce et al. 2005). In the case of the [Het-s] prion, it has been shown that the potential prion-inducing properties of the PFD can be modulated by the PFD's interaction with its related globular domain (Balguerie et al. 2003). To explain the loss of prion-inducing properties of the PFDSp when fused to the C-terminal domain, it is tempting to speculate that this is due to the N79D change because asparagine-to-aspartate mutations were shown to have a drastic effect on [PSI+] induction (Osherovich and Weissman 2001). However, this mutation is also present in the PFDSu sequence with no dramatic consequences. Another possibility to explain the loss of prion properties in Ure2pSp could be that the longer asparagine stretch and/or the change of a few amino acids couples the PFD to the C-terminal domain in a manner that would prevent [URE3] apparition. In the same way, overexpression of PFDs that usually induce [URE3] in S. cerevisiae and S. uvarum species (this study and Baudin-Baillieu et al. 2003) does not lead to such an induction in S. paradoxus probably because the PFD of Ure2p embedded in the whole protein is unable to interact with overexpressed PFD and allow prion propagation. In conclusion, the prion property is linked to the whole protein and is not restricted to its sole PFD.
[URE3] as an evolutionary significant epigenetic metabolic switch?:
While prions are lethal pathogens in mammals (for review see Dobson 1999), their physiological meaning is different in other organisms. For instance, in the case of the [Het-s] prion of the fungus P. anserina, the prion form of the protein is the active form in the cell-cell recognition phenomenon that might be beneficial for that species (Coustou et al. 1997). Also, several studies support the idea that [PSI+] confers some advantage to the cells harboring it (Eaglestone et al. 1999; True and Lindquist 2000; Namy et al. 2002). It has also been proposed that the metastable [PSI+] state offers the opportunity for emergence of new traits (True et al. 2004). Conservation of the prion properties throughout evolution among the various Sup35p orthologs supports this hypothesis (Chernoff et al. 2000; Kushnirov et al. 2000; Santoso et al. 2000; Jensen et al. 2001; Nakayashiki et al. 2001; Resende et al. 2002, 2003). Concerning [URE3], the situation described here reveals a more complex relationship between genes and phenotypes. Sequences of the PFDs evolved more quickly than those of the globular domain in Ure2p (Baudin-Baillieu et al. 2003). Moreover, prion property is lost or retained without correlation with the importance of these variations. Surprisingly, S. uvarum retains the prion function whereas S. paradoxus does not, although as compared to S. cerevisiae, PFDSu shows a larger difference in sequence than PFDSp (Figure 3A). To explain this paradox, it can be hypothesized that a selective pressure acts on the prion property in S. cerevisiae and S. uvarum, but not in S. paradoxus. Two facts are in agreement with this idea:
S. cerevisiae and S. uvarum are found in the same natural biotope, often in composite populations (Naumov et al. 2000). Conversely, S. paradoxus has never been found in these biotopes (I. Masneuf, personnal communication). It is thus reasonable to propose that the selective pressure, whatever it is, could act on S. cerevisiae and S. uvarum but not on S. paradoxus.
Δure2 strains of S. cerevisiae have been reported to be more selectively competitive on natural substrate compared to wild-type strains (Salmon and Barre 1998). The same kind of behavior would be expected for [URE3] strains since they have the same phenotype regarding the NCR.
Altogether, this suggests that: (1) there is a loose relation between amino acid sequence and prion property (Ross et al. 2004) and (2) a high rate of variations of those sequences can lead to this original evolution story.
Acknowledgments
We thank S. Saupe, M. Blondel, B. Daignan-Fornier, I. Sagot, and C. Schwimmer for helpful discussions and critical reading of the manuscript. We thank Mohan Gupta for looking over the English. We are grateful to R. B. Wickner and H. Edskes for generously providing pH660 plasmid. This work was supported by a grant from the Groupement d'Intérêt Scientifique “Infections à Prion” and the Fondation pour la Recherche Médicale. N.T. was supported by grants from the French Ministère de la Recherche and from the Centre National de la Recherche Scientifique.
References
- Aigle, M., and F. Lacroute, 1975. Genetical aspects of [URE3], a non-mitochondrial, cytoplasmically inherited mutation in yeast. Mol. Gen. Genet. 136: 327–335. [DOI] [PubMed] [Google Scholar]
- Bach, S., N. Talarek, T. Andrieu, J. M. Vierfond, Y. Mettey et al., 2003. Isolation of drugs active against mammalian prions using a yeast-based screening assay. Nat. Biotechnol. 21: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Bai, M., J. M. Zhou and S. Perrett, 2004. The yeast prion protein Ure2 shows glutathione peroxidase activity in both native and fibrillar forms. J. Biol. Chem. 279: 50025–50030. [DOI] [PubMed] [Google Scholar]
- Balguerie, A., S. Dos Reis, C. Ritter, S. Chaignepain, B. Coulary-Salin et al., 2003. Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J. 22: 2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin-Baillieu, A., E. Fernandez-Bellot, F. Reine, E. Coissac and C. Cullin, 2003. Conservation of the prion properties of Ure2p through evolution. Mol. Biol. Cell 14: 3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset, L., H. Belrhali, J. Janin, R. Melki and S. Morera, 2001. Structure of the globular region of the prion protein Ure2 from the yeast Saccharomyces cerevisiae. Structure 9: 39–46. [DOI] [PubMed] [Google Scholar]
- Bradley, M. E., H. K. Edskes, J. Y. Hong, R. B. Wickner and S. W. Liebman, 2002. Interactions among prions and prion “strains” in yeast. Proc. Natl. Acad. Sci. USA 99 (Suppl. 4): 16392–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, M. E., I. McConnell, H. Fraser and A. G. Dickinson, 1991. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J. Gen. Virol. 72: 595–603. [DOI] [PubMed] [Google Scholar]
- Chernoff, Y. O., A. P. Galkin, E. Lewitin, T. A. Chernova, G. P. Newnam et al., 2000. Evolutionary conservation of prion-forming abilities of the yeast Sup35 protein. Mol. Microbiol. 35: 865–876. [DOI] [PubMed] [Google Scholar]
- Cliften, P., P. Sudarsanam, A. Desikan, L. Fulton, B. Fulton et al., 2003. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science 301: 71–76. [DOI] [PubMed] [Google Scholar]
- Collinge, J., 2001. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24: 519–550. [DOI] [PubMed] [Google Scholar]
- Cooper, T. G., 2002. Transmitting the signal of excess nitrogen in Saccharomyces cerevisiae from the Tor proteins to the GATA factors: connecting the dots. FEMS Microbiol. Rev. 26: 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano, P. W., and B. Magasanik, 1991. The URE2 gene product of Saccharomyces cerevisiae plays an important role in the cellular response to the nitrogen source and has homology to glutathione s-transferases. Mol. Cell. Biol. 11: 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustou, V., C. Deleu, S. Saupe and J. Begueret, 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. USA 94: 9773–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, B. S., 1965. Psi, a cytoplasmic suppressor of super-suppressor in yeast. Heredity 20: 505–521. [Google Scholar]
- Cullin, C., and S. L. Minvielle, 1994. Multipurpose vectors designed for the fast generation of N- or C-terminal epitope-tagged proteins. Yeast 10: 105–112. [DOI] [PubMed] [Google Scholar]
- Derkatch, I. L., Y. O. Chernoff, V. V. Kushnirov, S. G. Inge-Vechtomov and S. W. Liebman, 1996. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C. M., 1999. Protein misfolding, evolution and disease. Trends Biochem. Sci. 24: 329–332. [DOI] [PubMed] [Google Scholar]
- Eaglestone, S. S., B. S. Cox and M. F. Tuite, 1999. Translation termination efficiency can be regulated in Saccharomyces cerevisiae by environmental stress through a prion-mediated mechanism. EMBO J. 18: 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes, H. K., and R. B. Wickner, 2002. Conservation of a portion of the S. cerevisiae Ure2p prion domain that interacts with the full-length protein. Proc. Natl. Acad. Sci. USA 99 (Suppl. 4): 16384–16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Bellot, E., E. Guillemet, A. Baudin-Baillieu, S. Gaumer, A. A. Komar et al., 1999. Characterization of the interaction domains of Ure2p, a prion-like protein of yeast. Biochem. J. 338: 403–407. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Bellot, E., E. Guillemet and C. Cullin, 2000. The yeast prion [URE3] can be greatly induced by a functional mutated URE2 allele. EMBO J. 19: 3215–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, J. R., A. S. Kowal, E. C. Schirmer, M. M. Patino, J. J. Liu et al., 1997. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89: 811–819. [DOI] [PubMed] [Google Scholar]
- Hawthorne, D., and P. Philippsen, 1994. Genetic and molecular analysis of hybrids in the genus Saccharomyces involving S. cerevisiae, S. uvarum and a new species, S. douglasii. Yeast 10: 1285–1296. [DOI] [PubMed] [Google Scholar]
- Herbert, C. J., M. Labouesse, G. Dujardin and P. P. Slonimski, 1988. The NAM2 proteins from S. cerevisiae and S. douglasii are mitochondrial leucyl-tRNA synthetases, and are involved in mRNA splicing. EMBO J. 7: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, D. G., J. D. Thompson and T. J. Gibson, 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266: 383–402. [DOI] [PubMed] [Google Scholar]
- Jensen, M. A., H. L. True, Y. O. Chernoff and S. Lindquist, 2001. Molecular population genetics and evolution of a prion-like protein in Saccharomyces cerevisiae. Genetics 159: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis, M., N. Patterson, M. Endrizzi, B. Birren and E. S. Lander, 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423: 241–254. [DOI] [PubMed] [Google Scholar]
- King, C. Y., and R. Diaz-Avalos, 2004. Protein-only transmission of three yeast prion strains. Nature 428: 319–323. [DOI] [PubMed] [Google Scholar]
- King, C. Y., P. Tittmann, H. Gross, R. Gebert, M. Aebi et al., 1997. Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc. Natl. Acad. Sci. USA 94: 6618–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnirov, V. V., N. V. Kochneva-Pervukhova, M. B. Chechenova, N. S. Frolova and M. D. Ter-Avanesyan, 2000. Prion properties of the Sup35 protein of yeast Pichia methanolica. EMBO J. 19: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddelein, M. L., and R. B. Wickner, 1999. Two prion-inducing regions of Ure2p are nonoverlapping. Mol. Cell. Biol. 19: 4516–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masison, D., and R. B. Wickner, 1995. Prion-inducing domain of yeast ure2p and protease resistance of ure2p in prion-containing cells. Science 270: 93–95. [DOI] [PubMed] [Google Scholar]
- Masison, D. C., M. L. Maddelein and R. B. Wickner, 1997. The prion model for [URE3] of yeast: spontaneous generation and requirements for propagation. Proc. Natl. Acad. Sci. USA 94: 12503–12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch, M. D., and J. S. Weissman, 2000. A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc. Natl. Acad. Sci. USA 97: 11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki, T., K. Ebihara, H. Bannai and Y. Nakamura, 2001. Yeast [PSI+] “prions” that are crosstransmissible and susceptible beyond a species barrier through a quasi-prion state. Mol. Cell 7: 1121–1130. [DOI] [PubMed] [Google Scholar]
- Namy, O., G. Duchateau-Nguyen and J. P. Rousset, 2002. Translational readthrough of the PDE2 stop codon modulates cAMP levels in Saccharomyces cerevisiae. Mol. Microbiol. 43: 641–652. [DOI] [PubMed] [Google Scholar]
- Naumov, G., I. Masneuf, E. Naumova, M. Aigle and D. Dubourdieu, 2000. Association of Saccharomyces bayanus var. uvarum with some French wines: genetic analysis of yeast populations. Res. Microbiol. 151: 683–691. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver, T. L., and J. W. Szostak, 1983. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc. Natl. Acad. Sci. USA 80: 4417–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherovich, L. Z., and J. S. Weissman, 2001. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI(+)] prion. Cell 106: 183–194. [DOI] [PubMed] [Google Scholar]
- Osherovich, L. Z., and J. S. Weissman, 2002. The utility of prions. Dev. Cell 2: 143–151. [DOI] [PubMed] [Google Scholar]
- Pierce, M. M., U. Baxa, A. C. Steven, A. Bax and R. B. Wickner, 2005. Is the prion domain of soluble Ure2p unstructured? Biochemistry 44: 321–328. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B., 1982. Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B., M. R. Scott, S. J. DeArmond and F. E. Cohen, 1998. Prion protein biology. Cell 93: 337–348. [DOI] [PubMed] [Google Scholar]
- Resende, C., S. N. Parham, C. Tinsley, P. Ferreira, J. A. Duarte et al., 2002. The Candida albicans Sup35p protein (CaSup35p): function, prion-like behaviour and an associated polyglutamine length polymorphism. Microbiology 148: 1049–1060. [DOI] [PubMed] [Google Scholar]
- Resende, C. G., T. F. Outeiro, L. Sands, S. Lindquist and M. F. Tuite, 2003. Prion protein gene polymorphisms in Saccharomyces cerevisiae. Mol. Microbiol. 49: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Ripaud, L., L. Maillet and C. Cullin, 2003. The mechanisms of [URE3] prion elimination demonstrate that large aggregates of Ure2p are dead-end products. EMBO J. 22: 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, E. D., U. Baxa and R. B. Wickner, 2004. Scrambled prion domains form prions and amyloid. Mol. Cell. Biol. 24: 7206–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, J. M., and P. Barre, 1998. Improvement of nitrogen assimilation and fermentation kinetics under enological conditions by derepression of alternative nitrogen-assimilatory pathways in an industrial Saccharomyces cerevisiae strain. Appl. Environ. Microbiol. 64: 3831–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso, A., P. Chien, L. Z. Osherovich and J. S. Weissman, 2000. Molecular basis of a yeast prion species barrier. Cell 100: 277–288. [DOI] [PubMed] [Google Scholar]
- Schlumpberger, M., S. B. Prusiner and I. Herskowitz, 2001. Induction of distinct [URE3] yeast prion strains. Mol. Cell. Biol. 21: 7035–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Sherman, F., and J. Hicks, 1991. Micromanipulation and dissection of asci. Methods Enzymol. 194: 21–37. [DOI] [PubMed] [Google Scholar]
- Sondheimer, N., and S. Lindquist, 2000. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell 5: 163–172. [DOI] [PubMed] [Google Scholar]
- Souciet, J., M. Aigle, F. Artiguenave, G. Blandin, M. Bolotin-Fukuhara et al., 2000. Genomic exploration of the hemiascomycetous yeasts. 1. A set of yeast species for molecular evolution studies. FEBS Lett. 487: 3–12. [DOI] [PubMed] [Google Scholar]
- Talarek, N., E. J. Louis, C. Cullin and M. Aigle, 2004. Developing methods and strains for genetic studies in Saccharomyces bayanus var. uvarum species. Yeast 21: 1195–1203. [DOI] [PubMed] [Google Scholar]
- Tanaka, M., P. Chien, N. Naber, R. Cooke and J. S. Weissman, 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428: 323–328. [DOI] [PubMed] [Google Scholar]
- Taylor, K. L., N. Cheng, R. W. Williams, A. C. Steven and R. B. Wickner, 1999. Prion domain initiation of amyloid formation in vitro from native Ure2p. Science 283: 1339–1343. [DOI] [PubMed] [Google Scholar]
- Ter-Avanesyan, M. D., A. R. Dagkesamanskaya, V. V. Kushnirov and V. N. Smirnov, 1994. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics 137: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thual, C., A. A. Komar, L. Bousset, E. Fernandez-Bellot, C. Cullin et al., 1999. Structural characterization of Saccharomyces cerevisiae prion-like protein Ure2. J. Biol. Chem. 274: 13666–13674. [DOI] [PubMed] [Google Scholar]
- True, H. L., and S. L. Lindquist, 2000. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407: 477–483. [DOI] [PubMed] [Google Scholar]
- True, H. L., I. Berlin and S. L. Lindquist, 2004. Epigenetic regulation of translation reveals hidden genetic variation to produce complex traits. Nature 431: 184–187. [DOI] [PubMed] [Google Scholar]
- Tuite, M. F., 2000. Yeast prions and their prion-forming domain. Cell 100: 289–292. [DOI] [PubMed] [Google Scholar]
- Tuite, M. F., and N. Koloteva-Levin, 2004. Propagating prions in fungi and mammals. Mol. Cell 14: 541–552. [DOI] [PubMed] [Google Scholar]
- Umland, T. C., K. L. Taylor, S. Rhee, R. B. Wickner and D. R. Davies, 2001. The crystal structure of the nitrogen regulation fragment of the yeast prion protein Ure2p. Proc. Natl. Acad. Sci. USA 98: 1459–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uptain, S. M., and S. Lindquist, 2002. Prions as protein-based genetic elements. Annu. Rev. Microbiol. 56: 703–741. [DOI] [PubMed] [Google Scholar]
- Uptain, S. M., G. J. Sawicki, B. Caughey and S. Lindquist, 2001. Strains of [PSI(+)] are distinguished by their efficiencies of prion-mediated conformational conversion. EMBO J. 20: 6236–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach, A., A. Brachat, R. Pohlmann and P. Philippsen, 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10: 1793–1808. [DOI] [PubMed] [Google Scholar]
- Wickner, R. B., 1994. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264: 566–569. [DOI] [PubMed] [Google Scholar]
- Zadorskii, S. P., V. Sopovaiu and S. G. Inge-Vechtomov, 2000. Prionization of the Pichia methanolica SUP35 gene product in the yeast Saccharomyces cerevisiae. Genetika 36: 1322–1329. [PubMed] [Google Scholar]