Abstract
Actin nucleation by the Arp2/3 complex is under tight control, remaining inactive until stimulation by nucleation-promoting factors (NPFs). Although multiple NPFs are expressed in most cell types, little is known about how they are coordinated and whether they perform similar or distinct functions. We examined genetic relationships among the four S. cerevisiae NPFs. Combining las17Δ with pan1-101 or myo3Δmyo5Δ was lethal at all temperatures, whereas combining pan1-101 with myo3Δmyo5Δ showed no genetic interaction and abp1Δ partially suppressed las17Δ. These data suggest that NPFs have distinct and overlapping functions in vivo. We also tested genetic interactions between each NPF mutant and seven different temperature-sensitive arp2 alleles and purified mutant Arp2/3 complexes to compare their activities. Two arp2 alleles with mutations at the barbed end were severely impaired in nucleation, providing the first experimental evidence that Arp2 nucleates actin at its barbed end in vitro and in vivo. Another arp2 allele caused partially unregulated (“leaky”) nucleation in the absence of NPFs. Combining this mutant with a partially unregulated allele in a different subunit of Arp2/3 complex was lethal, suggesting that cells cannot tolerate high levels of unregulated activity. Genetic interactions between arp2 alleles and NPF mutants point to Abp1 having an antagonistic role with respect to other NPFs, possibly serving to attenuate their stronger activities. In support of this model, Abp1 binds strongly to Arp2/3 complex, yet has notably weak nucleation-promoting activity and inhibits Las17 activity on Arp2/3 complex in a dose-responsive manner.
NUCLEATION of actin filament assembly is a key control point in the regulation of actin network formation. The Arp2/3 complex is one of two cellular factors (the other being formins) that recently have emerged as conserved actin nucleators in virtually all eukaryotes (Welch and Mullins 2002; Wallar and Alberts 2003). Actin nucleation by the Arp2/3 complex is critical for many cellular processes, including endocytosis, intracellular transport, and leading edge advancement during cell migration. The Arp2/3 complex is composed of seven subunits: Arp3, Arp2, p40/ARPC1, p35/ARPC2, p21/ARPC3, p19/ARPC4, and p15/ARPC5 (Machesky et al. 1994). Arp2 and Arp3 are ∼46 and 39% identical to actin, respectively (Lees-Miller et al. 1992; Schwob and Martin 1992; Huang et al. 1996), and are predicted to directly nucleate actin polymerization. This is predicted on the basis of high degree of sequence and structural homology among Arp2, Arp3, and conventional actin, particularly at the barbed end of these proteins, i.e., subdomains I and III (Kelleher et al. 1995; Robinson et al. 2001). However, this has not yet been demonstrated experimentally.
Purified Arp2/3 complex alone poorly nucleates actin assembly and requires the addition of a nucleation-promoting factor (NPF) for efficient nucleation. NPFs include members of the Wiskott-Aldrich syndrome protein (WASp)/SCAR/WAVE family, cortactin, Abp1, Pan1, and fungal type I myosins (Goode and Rodal 2001; Welch and Mullins 2002). In the crystal structure of bovine Arp2/3 complex, Arp2 and Arp3 are well separated, suggesting that it is an inactive conformation (Robinson et al. 2001). Addition of WASp to Arp2/3 complex induces major structural rearrangements that drive Arp2 and Arp3 closer together, presumably to form a pseudoactin dimer that provides a template for actin nucleation (Goley et al. 2004; Rodal et al. 2005a). In addition to stabilizing this closed Arp2/3 conformation, WASp must present an actin monomer to the barbed ends of Arp2 and/or Arp3 (Welch and Mullins 2002).
One question that has arisen from the identification of multiple NPFs is whether they have similar or distinct cellular functions and mechanisms of activating the Arp2/3 complex. Further, it is unclear how their activities are coordinated in vivo. Even in a relatively simple eukaryote like Saccharomyces cerevisiae, five different NPFs are expressed: Las17/Bee1, the homolog of WASp; Abp1; Pan1, the homolog of Eps15; and Myo3 and Myo5, two type 1 myosins (Li 1997; Evangelista et al. 2000; Lechler et al. 2000; Duncan et al. 2001; Goode et al. 2001). All of these NPFs localize to and function at cortical actin patches, which are dynamic sites of actin assembly and endocytosis, but their individual roles have remained elusive. Some NPFs, including cortactin and Abp1, are known to bind to actin filaments and lack any detectable actin monomer-binding affinity (Goode et al. 2001; Uruno et al. 2001; Weaver et al. 2001). These specialized properties have raised the possibility that Abp1 and cortactin may perform NPF functions distinct from members of the WASp/SCAR/WAVE family. Indeed, one recent study showed that cortactin displaces WASp from Arp2/3 complex specifically in the presence of actin filaments (Uruno et al. 2003). Thus, cortactin may help recycle WASp after nucleation has occurred, making WASp available for new rounds of nucleation and stabilizing the actin filament branch point created by the initial nucleation event (Weaver et al. 2002). In yeast, Las17/WASp and Pan1 show similar timing of appearance on newly forming cortical actin patches, whereas Myo3/Myo5 and Abp1 appear later in patch development (Kaksonen et al. 2003; Huckaba et al. 2004; Jonsdottir and Li 2004). Together, these studies suggest that different NPFs may act either in parallel or in series to control Arp2/3 complex activities.
To more clearly define the relationships among NPFs in S. cerevisiae, we tested all pairwise combinations of NPF mutants for genetic interactions. In addition, we crossed each NPF mutant to seven different conditional alleles of arp2. We then purified Arp2/3 complex from several of the arp2 mutant strains and compared their ability to promote actin nucleation to determine the basis of their differential genetic interactions. Two distinct classes of arp2 mutant activities were revealed: nucleation-impaired and unregulated or “leaky” nucleation (i.e., promote actin assembly in the absence of NPFs). Our analyses of the nucleation-impaired mutants demonstrate the importance of barbed end actin nucleation by Arp2 for proper function of the Arp2/3 complex in vitro and in vivo. Differential genetic interactions with NPFs were observed for the two classes of arp2 alleles. These and other data suggest that Las17, Pan1, and Myo3/Myo5 have distinct yet overlapping roles in promoting Arp2/3 complex-dependent actin nucleation in vivo, whereas Abp1 may attenuate the activities of the stronger NPFs.
MATERIALS AND METHODS
Yeast strains, plasmid constructions, and DNA manipulations:
S. cerevisiae strains used in this study are listed in Table 1. Standard methods were employed for DNA manipulations, growth, and transformation of yeast (Guthrie and Fink 1991). For suppression analyses, abp1Δ las17Δ and arp2 abp1Δ double-mutant strains were transformed with a low-copy (CEN) plasmid expressing Abp1, pABP1 (Lila and Drubin 1997), or empty vector (pRS315), grown to saturation in selective media, and then plated in 10-fold serial dilutions on selective media and grown for 3 days at the indicated temperature. The pML9 vector (Longtine et al. 1998) was modified to include a TEV protease recognition site, generating pML9T. This vector was used to integrate a tag consisting of an in-frame TEV protease recognition site followed by three copies of the hemagglutin (HA) epitope at the C termini of the ARP3 and ARC18 genes, producing BGY623 (ARP3∷TEV-3HA∷HIS3mX6) and BGY694 (ARC18∷TEV-3HA∷HIS3mX6). pML9T was used by the Drubin lab to produce BGY741 (ARC15∷TEV-3HA∷HIS3mX6), a strain that they provided for us.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype (all S288c background) | Source |
|---|---|---|
| BGY134 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, ARP2∷URA3 | Madania et al. (1999) |
| BGY136 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-1∷URA3 | Madania et al. (1999) |
| BGY137 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-2∷URA3 | Madania et al. (1999) |
| BGY138 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-3∷URA3 | Madania et al. (1999) |
| BGY139 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-4∷URA3 | Madania et al. (1999) |
| BGY140 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-5∷URA3 | Madania et al. (1999) |
| BGY141 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-6∷URA3 | Madania et al. (1999) |
| BGY142 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-7∷URA3 | Madania et al. (1999) |
| BGY623 | MATa, ade2-1, his3,11-15, leu2-3,112, ura3-52, ARP3-TEV3HA∷HIS3mX6 | This study |
| BGY694 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52 ARC18-TEV3HA∷HIS3mX6 | Rodal et al. (2005b) |
| BGY741 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, ARC15-TEV3HA∷HIS3mX6 | Drubin lab |
| BGY807 | MATa, his3,11-15, leu2-3,112,TRP1, LYS2, ura3-52, arc35Δ∷KANmX, LEU2∷arc35-5∷LEU2 | Rodal et al. (2005a) |
| BGY809 | MATa, his3,11-15, leu2-3,112,TRP1, LYS2, ura3-52, arc35Δ∷KANmX, LEU2∷arc35-6∷LEU2 | Rodal et al. (2005a) |
| BGY915 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, ARP2∷URA3, ARC18-TEV3HA∷HISmX6 | This study |
| BGY916 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-2∷URA3, ARC18-TEV3HA∷HISmX6 | This study |
| BGY917 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-5∷URA3, ARC18-TEV3HA∷HISmX6 | This study |
| BGY918 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-6∷URA3, ARC18-TEV3HA∷HISmX6 | This study |
| BGY919 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-7∷URA3, ARC18-TEV3HA∷HISmX6 | This study |
| BGY920 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, ARP2∷URA3, ARC15-TEV3HA∷HISmX6 | This study |
| BGY921 | MATα, ade2-1, his3,11-15, leu2-3,112, trp1-1, ura3-52, arp2-1∷URA3, ARC15-TEV3HA∷HISmX6 | This study |
| BGY924 | MATα, his3Δ200, leu2-3,112, lys2-801(oc), ura3-52, pan1-101∷LEU2 | This study |
| DDY2859 | MATa, his3Δ200, leu2-3,112, lys2-801(oc), ura3-52, PAN1∷LEU2 | Drubin lab |
| DDY2860 | MATa, his3Δ200, leu2-3,112, lys2-801(oc), ura3-52, pan1-101∷LEU2 | Drubin lab |
| HA31-9C | MATa, ade2-201, can1-100, his3,11-15, leu2-3,112, trp1-1(am), ura3-52, myo3Δ∷HIS3, myo5Δ∷TRP1 | Andersonet al. (1998) |
| MDY6-1B | MATα, ade1-101, his3Δ200, leu2-3,112, lys2-801(oc), trp1-1, ura3-52, las17Δ∷URA3 | Drubin lab |
Strains BGY741, DDY2859, DDY2860, and MDY6-1B were provided by the Drubin laboratory.
Antibodies and immunoblotting:
Rabbit polyclonal antibody against yeast tubulin was a gift from Frank Solomon (Massachusetts Institute of Technology) and was used to demonstrate equal protein loading on immunoblots (e.g., Figure 2B). Rabbit polyclonal antibody against Arp2 (Moreau et al. 1996) was a gift from Barbara Winsor (Centre National de Recherche Scientifique, Université Louis Pasteur, Strasbourg, France). Chicken polyclonal antibodies were raised against S. cerevisiae actin and the VCA fragment of Las17 (Aves; Tigard, OR). Antibodies were used for immunoblotting at 1:20,000 (actin), 1:4,000 (VCA), 1:20,000 (tubulin), and 1:1000 (Arp2). HRP-conjugated secondary antibodies were used at 1:10,000, and blots were developed with ECL (AP Biotech).
Figure 2.
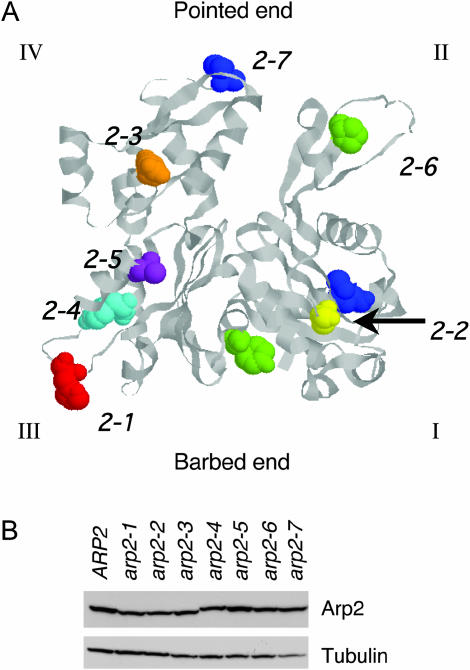
Positions of residues mutated in arp2 alleles and Arp2 expression levels in the mutant strains. (A) The residues mutated in each arp2 allele (Table 1) are highlighted on the crystal structure of rabbit skeletal muscle actin (1ATN). The crystal structure of Arp2 is not shown because only half of its structure is solved (Robinson et al. 2001). The four subdomains (I, II, III, and IV) are indicated, and the Arp2-specific loop in subdomain III was inserted from a crystallized portion of Arp2 (1K8K). The specific residues mutated in each allele are shown in space-fill and color-coded: arp2-1 (red), arp2-2 (yellow), arp2-3 (orange), arp2-4 (cyan), arp2-5 (purple), arp2-6 (green), and arp2-7 (blue). (B) Arp2 protein levels were compared in wild-type and arp2 mutant cells grown to log phase by immunoblotting total cell extracts with Arp2 antibodies. The same samples were immunoblotted with tubulin antibodies as a loading control. Although some lanes on the tubulin blot show a lower signal (e.g., arp2-7), repeated experiments demonstrated that variation was due to inefficient transfer of some lanes and that tubulin and Arp2 levels are similar in whole-cell extracts from these strains (not shown).
Cell-free actin assembly assay:
Frozen yeast cells were lysed by mechanical shearing in liquid nitrogen (Goode 2002). HE buffer (20 mm HEPES, pH 7.5, 1 mm EDTA) and protease inhibitors (final concentration of 0.5 μg/ml each of antipain, leupeptin, pepstatin A, chymostatin, aprotinin, and 1 mm PMSF) were then added to a 1:1 ratio (v/w). The thawed lysates were clarified by centrifugation at 4°, 20 min at 80,000 rpm in a TLA100.3 rotor (Beckman, Fullerton, CA). The resulting high-speed supernatants (HSS) were normalized after measuring protein concentration by two methods: Bradford assay and comparison of samples on Coomassie-stained gels. Normalized HSS fractions were incubated at 4° for 2 hr to allow actin to assemble. Actin in polymer was pelleted by centrifuging the reactions at 80,000 rpm for 5 min in a TLA100 rotor at 4°. Supernatants and pellets were fractionated on SDS-PAGE gels, immunoblotted with actin antibodies. Signals were quantified by scanning densitometry.
Protein purification:
Arp2/3 complex was isolated from wild-type and arp2 mutant strains with TEV-HA epitope tags integrated at the C terminus of the gene encoding the Arp3, Arc18/ARPC3, or Arc15/ARPC5 subunit. For a single preparation, 4 liters of yeast culture in YPD were grown to OD600 = 0.8, washed, resuspended in a 3:1 ratio (w/v) of cell pellet to H2O, and frozen in liquid nitrogen. Cells were lysed as above, and then the frozen cell powder was thawed in a 1:1 (w/v) ratio of room temperature HEK buffer (20 mm HEPES, pH 7.5, 1 mm EDTA, 50 mm KCl) and protease inhibitors (as above). Lysate (15 ml) was centrifuged for 20 min at 80,000 rpm (260,000 × g) in a TLA100.3 rotor. The supernatant was incubated with 100 μl of protein A-CL4B (AP Biotech) beads bound to anti-HA antibodies (HA.11; Covance) (Goode et al. 2001). After 2 hr of incubation, the beads were washed three times with HEK (1.5 ml), once with 1.5 ml HEKT (20 mm HEPES, pH 7.5, 1 mm EDTA, 50 mm KCl, 0.5% v/v Triton X-100), two times with 1.5 ml HEK500 (20 mm HEPES, pH 7.5, 1 mm EDTA, 500 mm KCl), and once with 1.5 ml HEK. Beads were then resuspended in 100 μl of HEK to create a 1:1 bead slurry. Two units of recombinant TEV protease (Invitrogen) were added, proteins were incubated for 1 hr at room temperature, and then two more units of TEV protease were added and the proteins were incubated for an additional hour. The supernatant was removed from the beads by low-speed centrifugation, aliquoted, and flash-frozen in liquid nitrogen. rTEV has no effect on Arp2/3 complex-mediated actin polymerization (Rodal et al. 2003).
Rabbit skeletal muscle actin (Pollard 1984), yeast actin (Goode 2002), full-length Las17 (Rodal et al. 2003), and Abp1-AN*C* (Goode et al. 2001) were purified as described. Wild- type Abp1 and Abp1ΔADFH were isolated from BJ2168 (Jones 2002) transformed with pGalABP1 or pGalABP1ΔADFH (Quintero-Monzon et al. 2005). High-level expression was induced with galactose as described (Rodal et al. 2002). A high-speed supernatant was generated as above using HEK buffer and applied to a HiTrap Q FF 5-ml anion exchange column (AP Biotech). Proteins were eluted with a linear gradient of KCl (0.2–0.6 m) in HEK buffer and 5% glycerol. Peak fractions were pooled, diluted to 0.1 m KCl using HE buffer, and loaded onto a Mono Q (5/5) column. Proteins were eluted with a linear gradient of KCl (0.25–0.60 m) in HEK buffer plus 5% glycerol. Peak fractions were concentrated to 300 μl and applied to a Superose-12 (10/30) gel filtration column equilibrated in HEK buffer. Peak fractions were concentrated to 15–75 μm, aliquoted, and flash frozen in liquid N2.
Actin assembly kinetics:
Rabbit skeletal muscle actin was labeled with pyrenyliodoacetamide as described (Pollard 1984; Higgs et al. 1999). Actin monomers were prepared by gel filtration using a Sephacryl S-200 column equilibrated in G-buffer (10 mm Tris, pH 8.0, 0.2 mm ATP, 0.2 mm CaCl2, and 0.2 mm DTT). Actin filament assembly assays were performed as described (Xu et al. 2004). Briefly, gel-filtered actin monomers (final concentration 3 μm, 5% pyrene labeled) were mixed with exchange buffer (10 mm EGTA, 1 mm MgCl2) for 2 min (Kovar et al. 2003) and then mixed with 10 μl of HEK buffer or proteins in HEK buffer. This mixture was added immediately to 3 μl of 20× initiation mix (40 mm MgCl2, 10 mm ATP, 1 m KCl) to initiate actin assembly. Pyrene fluorescence was monitored at excitation 365 nm and emission 407 nm in a fluorescence spectrophotometer (Photon Technology International) at 25°. The rate of polymerization was calculated from the slopes of assembly curves at ∼20–50% polymerization. The number of free barbed ends was calculated as described (Pollard 1986).
RESULTS
Genetic relationships among yeast nucleation-promoting factors:
To begin to dissect the in vivo relationships among S. cerevisiae NPFs, we tested all pairwise genetic interactions among las17Δ, pan1-101, myo3Δmyo5Δ, and abp1Δ mutations. Directed crosses were performed between haploid strains bearing these mutations. Diploids were selected, sporulated, and the resulting haploid progeny (wild type, single mutants, and double mutants) were compared for growth at 25°, 30°, 34°, and 37°. The resulting genetic interactions are diagrammed in Figure 1A. Combining las17Δ with myo3Δmyo5Δ was lethal at all temperatures, consistent with previous studies (Evangelista et al. 2000; Lechler et al. 2000). In contrast, pan1-101, which carries a mutation in its acidic motif required for Arp2/3 complex activation, had a genetic interaction with las17Δ, but not myo3Δmyo5Δ. These data suggest that Las17 and type I myosins have distinct yet overlapping functions in vivo, consistent with the partial overlap in their temporal patterns of association with actin patches (Jonsdottir and Li 2004 and see discussion).
Figure 1.
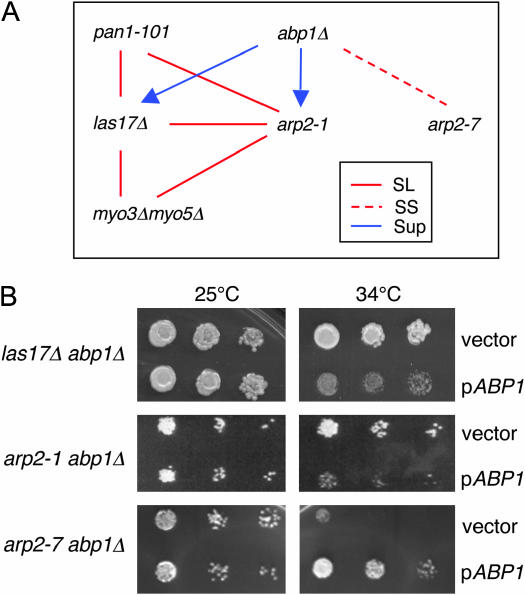
Genetic interactions among nucleation-promoting factor mutants and arp2 alleles. (A) Diagram of genetic relationships. Six haploid strains bearing las17Δ, abp1Δ, myo3Δ myo5Δ, pan1-101, arp2-1, and arp2-7 mutations were crossed in all pairwise combinations to test genetic interactions. The data for arp2 alleles are summarized in Table 2. The absence of a line indicates that no interaction was detected. SL, synthetic lethal; SS, synthetic sick; SUP, suppression. The direction of the arrow indicates direction of suppression. Thus, the growth defects of both arp2-1 and las17Δ strains are partially suppressed by abp1Δ. (B) Differential genetic interactions of abp1Δ with las17Δ, arp2-1, and arp2-7 mutations. Haploid abpΔ las17Δ, abp1Δ arp2-1, and abp1Δ arp2-7 strains were transformed with a pABP1 CEN expression plasmid (Lila and Drubin 1997) or empty vector, plated on selective media, and grown at 25° and 34°.
Interestingly, combining abp1Δ with other NPF mutations (las17Δ, pan1-101, and myo3Δmyo5Δ) did not cause any synthetic phenotypes. Instead, abp1Δ suppressed the growth defects of las17Δ (Figure 1A), improving growth at 25° and permitting growth at the restrictive temperature (34°), which was never observed for las17Δ cells. Suppression also was observed independently in isogenic strains by comparing the growth of a las17Δ abp1Δ strain transformed with either a low-copy ABP1 expression plasmid or empty vector (Figure 1B). Thus, in two different assays, las17Δ abp1Δ cells grew more robustly than las17Δ cells. These data point to a unique function for Abp1, possibly antagonistic to other NPFs.
Genetic interactions among nucleation-promoting factors and arp2 alleles:
We reasoned that we may be able to separate further the roles of NPFs using a collection of mutant alleles in arp2, for which differential genetic interactions with las17Δ were reported previously (Madania et al. 1999). The seven conditional arp2 alleles were generated by random mutagenesis, and each allele contains either one or two single-point mutations (Moreau et al. 1996). The positions of the residues mutated in each allele are modeled on the crystal structure of actin to highlight their approximate positions in Arp2 (Figure 2A). We found that all seven arp2 alleles have defects in both actin organization and endocytosis (not shown), consistent with the original study (Moreau et al. 1996). It also had been reported that all of the alleles except for arp2-7 are lethal in combination with las17Δ at 25°, and the temperature-sensitive growth defects of arp2-1 and arp2-2 are suppressed by overexpression of Las17 (Madania et al. 1999). This suggested that the alleles might provide excellent tools for separating NPF functions.
We crossed each NPF mutant strain (las17Δ, abp1Δ, myo3Δ myo5Δ, and pan1-101) to strains expressing each temperature-sensitive arp2 allele (arp2-1 through arp2-7). The genetic interactions we observed between arp2 alleles and las17Δ are consistent with previous work (Madania et al. 1999). Additionally, we identified new genetic interactions between arp2 alleles and myo3Δmyo5Δ, pan1-101, and abp1Δ (Table 2).
TABLE 2.
Genetic interactions between arp2 alleles and nucleation-promoting factors
| Allele | Mutation(s)a | LAS17 OEb | las17Δb | abp1Δ | myo3Δ, myo5Δ | pan1-101 |
|---|---|---|---|---|---|---|
| ARP2 | NE | NE | NE | NE | NE | |
| arp2-1 | H330L | Sup | SL | Sup | SL | NE |
| arp2-2 | G19D | Sup | SL | Sup | SL | Sup |
| arp2-3 | E256K | NE | SL | NE | NE | SS |
| arp2-4 | L280P | NE | SL | SL | NE | Sup |
| arp2-5 | L316S | NE | SL | SS | NE | SS |
| arp2-6 | G35S, Y144N | NE | SL | SS | NE | NE |
| arp2-7 | F127S, F203Y | NE | NE | SS | NE | NE |
NE, no effect; SL, synthetic lethal; SS, synthetic sick; Sup, suppression (growth at 37° for double mutants, which was never observed for single mutants).
Data from Moreau et al. (1996).
Data from Madania et al. (1999).
Two alleles, arp2-1 and arp2-2, were synthetic lethal with las17Δ and myo3Δmyo5Δ. These data support the genetic grouping of arp2-1 and arp2-2 and are consistent with the observation that LAS17 and MYO3/MYO5 share overlapping genetic functions (above). In contrast, arp2-7 showed no genetic interactions with las17Δ or myo3Δmyo5Δ and was synthetic sick with abp1Δ. We demonstrated independently the specificity of this genetic interaction between arp2-7 and abp1Δ in an isogenic background by comparing the growth of arp2-1 abp1Δ and arp2-7 abp1Δ strains transformed with an ABP1 plasmid or empty vector (Figure 1B). The temperature sensitivity caused by arp2-1 was rescued by abp1Δ. Together, these data place arp2-7 in a distinct genetic category from arp2-1 and arp2-2. The remaining arp2 alleles (arp2-3, arp2-4, arp2-5, and arp2-6) showed complex patterns of genetic interactions that were difficult to interpret. This is likely to result from these alleles having multiple defects in Arp2/3 complex activities and functions. Therefore, we focused primarily on arp2-1, arp2-2, and arp2-7 in our further analyses.
The patterns of genetic interactions observed between arp2 alleles and NPF mutants neither correlate with differences in the severity of growth defects caused by the mutations (not shown) nor result from differences in Arp2 expression levels (Figure 2B). The specificity of the genetic interactions prompted us to investigate the underlying biochemical basis of the arp2 defects.
Isolation of wild-type and arp2 mutant Arp2/3 complexes:
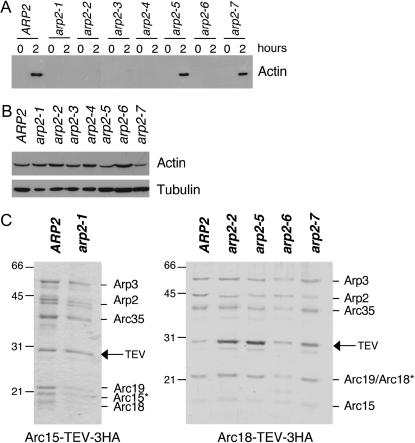
For a fair comparison of their activities, it was important to isolate Arp2/3 complex from wild-type, arp2-1, arp2-2, and arp2-7 strains by the same method. Initially, we used our previously described method for isolating wild-type yeast Arp2/3 complex, which relies on an initial step of reconstituting actin assembly in clarified yeast cell lysates at 4° (Goode 2002). This assay cannot be performed at higher temperatures due to proteolysis effects. Recently, we determined that actin assembly in this assay requires Arp2/3 complex and Las17, but not other NPFs (A. Goodman, J. L. D'Agostino and B. L. Goode, unpublished results). Therefore, it stands to reason that actin assembly will fail in cell extracts from arp2 mutants with severely compromised actin nucleation activity. As shown in Figure 3A, actin assembly failed in cell extracts from five of the seven arp2 strains, despite the fact that they had comparable levels of both actin (Figure 3B) and Arp2 (Figure 2B). In particular, we noted that cell extracts from arp2-1 and arp2-2 strains failed to polymerize actin, whereas extracts from arp2-7 cells polymerized actin like wild-type extracts. These data are consistent with the differential genetic interactions of these arp2 alleles with LAS17 and provided the first indication of key differences in the biochemical defects of arp2-1 and arp2-2 compared to arp2-7.
Figure 3.
Purification of Arp2/3 complex from wild-type and arp2 mutant strains. (A) Clarified cell extracts isolated from wild-type and arp2 strains were compared for their ability to nucleate actin assembly over time. Samples of reactions were removed at time zero and after 2 hr of incubation at 4° and centrifuged at 80,000 × g to pellet polymerized actin (Goode 2002). Actin levels in the pellets were analyzed by SDS-PAGE and immunoblotting with actin antibodies; >50% of the cellular actin assembles in wild-type extracts (A. Goodman, J. L. D'Agostino and B. L. Goode, unpublished results). (B) Starting actin levels in the clarified extracts were compared by immunoblotting with actin antibodies. The same samples were immunoblotted with tubulin antibodies as a loading control. (C) Coomassie-stained gels of Arp2/3 complex isolated from wild-type (ARP2) and arp2 strains. A TEV-3xHA affinity tag integrated at the C terminus of the Arc15/ARPC5 subunit was used to isolate Arp2/3 complex from wild-type and arp2-1 strains. A similar tag on the Arc18/ARPC3 subunit was used to isolate Arp2/3 complex from wild-type, arp2-2, arp2-5, arp2-6, and arp2-7 strains. A remnant of nine residues is left on the indicated subunit after TEV protease digestion, causing the aberrant migration of that subunit (*).
Since the cell extract method could not be used to isolate Arp2/3 complex from arp2-1 and arp2-2 strains, we developed an alternative strategy that employs an integrated C-terminal affinity tag on one subunit of the Arp2/3 complex. The tag consists of a TEV protease recognition site followed by three copies of the hemagglutin (HA) epitope, and importantly, it is removed from Arp2/3 complex during purification, leaving only a short remnant on one subunit (see materials and methods). Prior to its removal though, the tag partially compromises Arp2/3 complex function in vivo, as indicated by the partially impaired growth of tagged strains at higher temperatures and synthetic genetic interactions between tagged subunits and arp2 alleles (Table 3). Tagging Arp2 in each of the seven arp2 strains resulted in lethality, indicating that this single subunit cannot function properly if both mutated and tagged. However, other tagged subunits (Arp3, Arc18/ARPC3, and Arc15/ARPC5) were found to be viable in combination with specific arp2 alleles: arp2-1, arp2-2, arp2-5, arp2-6, and arp2-7 alleles. Thus, an Arc18/ARPC3 tag was used to isolate Arp2/3 complex from wild-type, arp2-2, arp2-5, arp2-6, and arp2-7 strains, and an Arc15/ARPC5 tag was used to isolate Arp2/3 complex from wild-type and arp2-1 strains (Figure 3C). All of the tagged subunits tested were synthetic lethal with arp2-3 and arp2-4 (Table 3), precluding the isolation of Arp2/3 complex from these strains.
TABLE 3.
Genetic interactions between arp2 alleles and tagged subunits of the Arp2/3 complex
| Allele | ARP2-T-3xHA | ARP3-T-3xHA | ARC18-T-3xHA | ARC15-T-3xHA |
|---|---|---|---|---|
| ARP2 | Viable | Viable | Viable | Viable |
| arp2-1 | SL | SL | SL | Viablea |
| arp2-2 | SL | Viablea | Viable | Viable |
| arp2-3 | SL | SL | SL | SL |
| arp2-4 | SL | SL | SL | SL |
| arp2-5 | SL | Viable | Viable | SL |
| arp2-6 | SL | SL | Viable | SL |
| arp2-7 | SL | Viable | Viable | SL |
Each subunit has at its carboxyl terminus an integrated tag consisting of a TEV protease cleavage site followed by three copies of the HA epitope. All of the viable haploid strains are underlined, and unless otherwise noted, viable haploid strains grow at 25° in rich medium. SL, synthetic lethal.
Viable at 25° when medium is supplemented with 0.9 M sorbitol.
Purified wild-type and arp2 mutant Arp2/3 complexes are shown fractionated on SDS-PAGE gels (Figure 3C). The positions of six of the seven subunits in the complex are labeled. Mass spectrometry analysis showed that the remaining subunit p40/ARPC1 is present (data not shown); however, this subunit in S. cerevisiae exhibits aberrant gel mobility (Pan et al. 2004). The remnant from the tag causes signature differences in the gel mobilities of Arc18/ARPC3 and Arc15/ARPC5 (asterisks, Figure 3C). However, the activity of wild-type Arp2/3 complex prepared using either tag (Arc18/ARPC3 or Arc15/ARPC5) was indistinguishable from tag-less wild-type Arp2/3 complex isolated by the extract method (not shown, and Rodal et al. 2003, 2005a). Further, we isolated arp2-7 Arp2/3 complex separately using two different tagged subunits (Arc18/ARPC3 and Arp3) and found their actin nucleation activities to be indistinguishable.
Distinct biochemical defects in the activities of arp2-1 and arp2-7 Arp2/3 complexes:
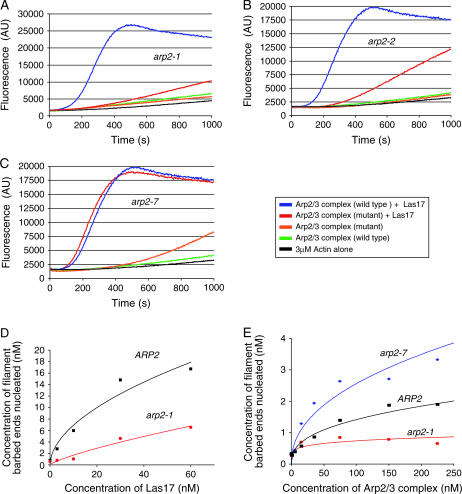
Next, we directly compared the actin nucleation capabilities of the wild-type and mutant arp2 Arp2/3 complexes (Figure 4). The pyrene-actin assembly assay was used to monitor assembly kinetics of purified actin monomers (3 μm) in the presence of wild-type or mutant Arp2/3 complex (15 nm) with and without full-length Las17 (15 nm). As expected, wild-type, arp2-1, and arp2-2 Arp2/3 complexes alone had little actin nucleation activity in the absence of Las17 (Figure 4, A and B). Addition of Las17 strongly stimulated wild-type Arp2/3 complex, but had minimal stimulatory effects on arp2-1 and arp2-2 Arp2/3 complexes, indicating that these mutations cause impaired actin nucleation. Given that LAS17 (a strong NPF) was identified as a multicopy suppressor of arp2-1 (Madania et al. 1999), we tested whether higher concentrations of Las17 could biochemically restore actin nucleation function to arp2-1 mutant Arp2/3 complex. Therefore, we compared the nucleation activities of wild-type and arp2-1 Arp2/3 complexes over a range of Las17 concentrations (3–60 nm). Addition of increasing Las17 concentrations increased the nucleation activity of arp2-1 Arp2/3 complex in a dose-responsive manner (Figure 4D). These data offer a biochemical explanation for why overexpression of Las17 can suppress arp2-1 defects in vivo. The biochemical defects of the purified arp2-1 and arp2-2 mutant Arp2/3 complexes are also consistent with the genetic interactions of these alleles with LAS17 (Table 2) and with the failure of actin to assemble in cell extracts from arp2-1 and arp2-2 strains (Figure 3A). Importantly, the data also show that arp2-1 Arp2/3 complex is impaired for actin nucleation, even in the absence of Las17. This suggests that a primary defect of arp2-1 is in directly nucleating actin, which is also consistent with the mutations in these alleles residing at the barbed end (Figure 2A).
Figure 4.
Actin nucleation activities of Arp2/3 complexes isolated from wild-type and mutant arp2 strains. (A–C) Assembly of monomeric actin (3 μm, 5% pyrene labeled) was compared in the presence and absence of 15 nm full-length Las17 and 15 nm wild type or arp2-1 (A), arp2-2 (B) or arp2-7 (C) Arp2/3 complex. (D) Comparison of the actin nucleation activities of wild-type and arp2-1 Arp2/3 complexes (15 nm) in the presence of variable concentrations of Las17. (E) Quantitative comparison of actin nucleation activities for wild-type, arp2-1, and arp2-7 Arp2/3 complexes in the absence of Las17.
In contrast to arp2-1 and arp2-2 mutants, arp2-7 Arp2/3 complex was activated normally by Las17, with activity similar to wild-type Arp2/3 complex (Figure 4C). However, arp2-7 Arp2/3 complex showed aberrantly high nucleation activity in the absence of Las17, suggesting that this allele causes unregulated (leaky) nucleation activity. To characterize this effect more carefully, we compared the nucleation activities of wild-type, arp2-1, and arp2-7 Arp2/3 complexes alone, in the absence of Las17, over a range of Arp2/3 complex concentrations (Figure 4E). This confirmed that arp2-7 Arp2/3 complex is auto-activated. We also note that arp2-7 Arp2/3 complex is not overactive compared to wild-type Arp2/3 complex in the presence of Las17 (Figure 4C), suggesting that arp2-7 is not a gain-of-function mutant (i.e., it does not alter Arp2 to improve its efficiency in nucleating actin). This is also consistent with our in vivo data; we detected no defects in cell growth or actin organization in heterozygous arp2-7 diploids (Table 4, and data not shown); thus, arp2-7 behaves as a recessive allele. On the basis of these results, we hypothesize that arp2-7 disrupts the normal auto-inhibition by Arp2/3 complex that minimizes nucleation in the absence of NPFs (see discussion). Below we refer to arp2-1 and arp2-7 as nucleation-impaired and constitutively active mutants, respectively.
TABLE 4.
Genetic interactions of arp2 alleles with arc35 alleles
| Genotype | 25° | 30° | 34° | 37° | Genetic interaction |
|---|---|---|---|---|---|
| Haploid | |||||
| ARP2, ARC35 | +++ | +++ | +++ | +++ | NA |
| arp2-1, ARC35 | +++ | ++ | + | − | NA |
| arp2-7, ARC35 | +++ | ++ | + | − | NA |
| ARP2, arc35-5 | +++ | +++ | ++ | ++ | NA |
| ARP2, arc35-6 | +++ | +++ | +++ | +++ | NA |
| arp2-1, arc35-5 | ++ | ++ | − | − | NE/SS |
| arp2-7, arc35-5 | − | − | − | − | SL |
| arp2-1, arc35-6 | ++ | − | − | − | SS |
| arp2-7, arc35-6 | +++ | ++ | + | − | NE |
| Diploid | |||||
| ARP2/ARP2 | +++ | +++ | +++ | +++ | NA |
| arp2-1/ARP2 | +++ | +++ | +++ | +++ | Recessive |
| arp2-7/ARP2 | +++ | +++ | +++ | +++ | Recessive |
| arp2-1/arp2-1 | +++ | ++ | + | − | NA |
| arp2-7/arp2-7 | +++ | ++ | + | − | NA |
| arp2-1/arp2-7 | +++ | ++ | + | − | NE |
Relative growth of yeast strains is expressed as +++, wild type; ++, partially compromised; +, severely compromised; and −, no growth. NA, not applicable; NE, no effect; SL, synthetic lethal; SS, synthetic sick.
Genetic relationships between constitutively active and nucleation-impaired Arp2/3 complex mutants:
Recently, we reported that another Arp2/3 complex mutant (arc35-5), like arp2-7, is partially constitutively active for nucleation (Rodal et al. 2005a). arc35-5 is a partially temperature-sensitive allele of ARC35, which encodes the p35/ARPC2 subunit of Arp2/3 complex. Both arp2-7 and arc35-5 have actin organization defects at 25° (depolarized patches) and impaired or arrested cell growth at 37° (not shown). This indicates that cells can tolerate partial levels of unregulated nucleation by Arp2/3 complex at lower temperatures. To test whether cells can tolerate potentially higher levels of auto-activation, we crossed the arc35-5 and arp2-7 haploid strains and control strains (Table 4). The results show that arp2-7 is synthetic lethal specifically with arc35-5, but not arc35-6, an allele that is nucleation impaired (Rodal et al. 2005a). Although we observed a slight negative genetic interaction between arp2-1 and arc35-5, this interaction was much less pronounced than the synthetic lethal interaction between the two constitutively active alleles arp2-7 and arc35-5. Together, these data suggest that combining two constitutively active mutations within the same Arp2/3 complex causes additive or synergistic negative effects in vivo. Thus, cells cannot tolerate a high level of unregulated actin nucleation by Arp2/3 complex.
We also tested whether constitutively active alleles (arp2-7 and arc35-5) might have the capacity to suppress nucleation-impaired alleles (arp2-1 and arc35-6). In other words, we asked if rendering the Arp2/3 complex partially unregulated (leaky) could rescue in vivo defects in nucleation, and vice versa. To address this question, we tested suppression by two criteria: (1) combining mutations in haploid strains and (2) testing complementation in diploid strains that are homozygous and heterozygous for constitutively active and nucleation-impaired alleles. As shown in Table 4, in no case was the mutant phenotypes suppressed. Thus, constitutively active mutations cannot rescue nucleation-impaired mutations, and vice versa.
Abp1 attenuates the nucleation-promoting activity of Las17 in a dose-responsive manner:
In light of the biochemical activities of arp2-1 and arp2-7, the genetic data in Table 2 suggest that Las17, Pan1, and Myo3/Myo5 cooperate to promote actin assembly in vivo, whereas Abp1 may act to suppress actin assembly by other NPFs. Three observations support this view: (1) abp1Δ is synthetic sick with arp2-7 (constitutively active) but suppresses arp2-1 (nucleation impaired); (2) las17Δ has synthetic interactions with arp2-1, but not arp2-7; and (3) abp1Δ suppresses las17Δ. Further, the biochemical properties of Abp1 are consistent with such a role as an attenuator. Abp1 has a relatively high affinity for Arp2/3 complex and yet also has notably weak nucleation-promoting activity (Goode et al. 2001), especially compared to full-length Las17 (Rodal et al. 2003).
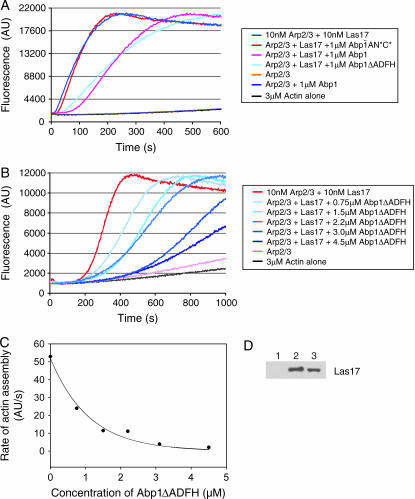
To test this model directly, we assayed the effects of purified Abp1 on Las17-Arp2/3 complex stimulated actin assembly in vitro (Figure 5). Abp1 has an N-terminal Actin Depolymerization Factor Homology (ADFH) domain that binds to actin filaments, whereas Las17 has a C-terminal WASp homology 2 (WH2) domain that associates with actin monomers. However, both Abp1 and Las17 associate with the Arp2/3 through conserved acidic motifs. Using an in vitro pyrene-actin assembly reaction, we tested the effects of adding Abp1 to reactions containing Las17 and Arp2/3 complex. Full-length wild-type Abp1 (1 μm) inhibited activation of Arp2/3 complex (15 nm) by Las17 (15 nm), but did not abolish it (Figure 5A). Abp1ΔADFH, which lacks nucleation-promoting activity, showed similar inhibitory effects, suggesting that inhibition may result from competition for Arp2/3 complex association by acidic motifs in Abp1 and Las17. Consistent with this hypothesis, a mutant of Abp1 (Abp1-AN*C*) that disrupts both of its Arp2/3-interacting acidic motifs (Goode et al. 2001) failed to inhibit Las17 (Figure 5A). Thus, functional competition between Abp1 and Las17 is dependent on Abp1 acidic motifs.
Figure 5.
Purified Abp1 attenuates the nucleation-promoting activity of Las17 on Arp2/3 complex in a dose-responsive manner. (A) Monomeric actin (3 μm, 5% pyrene-labeled) was polymerized in the presence of Arp2/3 complex (15 nm), Las17 (15 nm), and 1μm of each of the following proteins: full-length wild-type Abp1, Abp1ΔADFH, and Abp1-AN*C* (mutated to disrupt both of its acidic motifs). (B) Monomeric actin (as above) was polymerized in the presence of Arp2/3 complex (15 nm), Las17 (15 nm), and a range of concentrations of Abp1ΔADFH. (C) Graph showing the concentration-dependent effects of Abp1ΔADFH on rate of actin assembly induced by Las17 and Arp2/3 complex. (D) Association of Las17 (30 nm) with Arp2/3 complex (15 nm) is partially disrupted in the presence of excess Abp1 (2 μm). Beads coated with Arc18-HA-tagged Arp2/3 complex or control beads were incubated for 10 min with Las17 in the presence or absence of Abp1. Bead pellets were immunoblotted with anti-VCA antibody: Lane 1, control beads; lane 2, Arp2/3 beads without Abp1; lane 3, Arp2/3 beads with Abp1.
To determine the half-maximal effect of Abp1 inhibition on Las17-Arp2/3, we repeated the assay over a range of Abp1 concentrations (0–4.5 μm) (Figure 5B). Abp1-ΔADFH was used for this determination because it inhibits Las17 as well as Abp1 but lacks nucleation-promoting activity, simplifying the interpretation of the results. As shown in Figure 5C, the half-maximal inhibition of Las17 was reached at 1 μm Abp1-ΔADFH. This demonstrates that Abp1 does in fact attenuate the activity of stronger NPFs in a dose-responsive manner. Although strong inhibition required significantly higher levels of Abp1 compared to Las17, we note that Abp1 is ≥10-fold more abundant in vivo than Las17 (A. Goodman, J. L. D'Agostino and B. L. Goode, unpublished results). Further, full-length Las17 has higher affinity than Abp1 for Arp2/3 complex (J. L. D'Agostino, unpublished observations).
We next tested whether the functional competition between Abp1 and Las17 results from Abp1 displacement of Las17 from Arp2/3 complex. We measured direct binding of purified Las17 to Arp2/3 complex (15 nm Arp2/3 complex and 30 nm Las17, similar concentrations to those used in the functional assays above) in the presence and absence of excess Abp1 (2 μm). Partial displacement was observed (Figure 5D), consistent with the partial functional competition at this concentration of Abp1 (Figure 5B). From these data, we propose that Abp1 interacts with Arp2/3 complex via its acidic domains, which disrupts important functional interactions between the acidic domain of Las17 and Arp2/3 complex. While this is sufficient to disrupt Las17 functional activation of Arp2/3 complex, it may or may not be sufficient to completely displace Las17 (physically) from Arp2/3 complex.
DISCUSSION
Las17, Pan1, and Myo3/Myo5 have overlapping roles in promoting Arp2/3 complex-dependent actin assembly in vivo:
All of our genetic and biochemical data collectively suggest that Las17, Pan1, and Myo3/Myo5 cooperate in vivo to promote Arp2/3 complex-mediated actin assembly. This is demonstrated by their overlapping yet specific patterns of synthetic lethal interactions with each other and with arp2 alleles that we found are biochemically impaired in actin nucleation. Cortical actin patch nucleation in vivo requires the Arp2/3 complex (Winter et al. 1999). However, our data show that only specific pairs of mutations among Las17, Pan1, and Myo3/Myo5 are synthetic lethal, suggesting that the Arp2/3 complex-stimulating activity of no single NPF is required in vivo. Instead, each NPF makes a specific and important contribution to the complex process of patch development/endocytosis, and these events involve the combined, partially redundant nucleation-promoting activities of multiple NPFs.
What might the overlapping physiological roles of NPFs be in these events? Until recently, it was unclear whether yeast NPFs acted in parallel or series. However, recent studies using dual-label imaging in live cells have shown that actin patches are the sites of endocytic internalization and mature in defined stages, marked by the arrival and departure of specific NPFs (Kaksonen et al. 2003; Huckaba et al. 2004; Jonsdottir and Li 2004). This allows us to put the genetic data obtained here into the context of cell function.
Las17 and Pan1 are present at early stages in the development of cortical actin patches (Kaksonen et al. 2003). Las17 is thought to play a central role in initiating Arp2/3-mediated actin assembly at patches on the basis of its potent nucleation-promoting activity in vitro (Rodal et al. 2003) and its severe loss-of-function phenotypes in vivo (Li 1997). Our genetic data suggest that Las17 and Pan1 may cooperate to activate Arp2/3 complex-mediated actin assembly, perhaps at this stage, which agrees with other recent reports (Toshima et al. 2005). Subsequently, Myo3/Myo5, Abp1, and Arp2/3 complex arrive at patches (Kaksonen et al. 2003; Jonsdottir and Li 2004). Myo3/Myo5 persists for a short window of time, during which it may promote membrane fission and endosome/patch internalization. We show that Las17 and Myo3/Myo5 are synthetic lethal, in agreement with previous studies (Evangelista et al. 2000; Lechler et al. 2000), suggesting that their functions may be overlapping at this stage in endocytosis. After scission, Pan1, Abp1, and Arp2/3 complex remain on patches as they begin to move inward rapidly, eventually to fuse with endosomal sorting compartments, whereas Las17 is left behind at the cell cortex (Kaksonen et al. 2003; Huckaba et al. 2004). These observations combined with our genetic data suggest that NPFs each have specialized yet intertwined roles in patch development. Specifically, our data suggest a role for Abp1 as an antagonist to other NPFs, possibly during scission, discussed below.
The studies above suggest that NPFs act both in parallel and in series during patch development. Assessment of the specific roles of each NPF requires consideration of which NPFs function together in physical complexes. Las17 and Myo3/Myo5 associate in cell extracts, and both proteins interact directly with Vrp1, which may link them (Evangelista et al. 2000). In addition, the SH3 domain-containing protein Sla1 associates with three different NPFs: Las17 (Li 1997; Rodal et al. 2003), Pan1 (Tang et al. 2000), and Abp1 (Gourlay et al. 2003). Thus, all four NPFs have the potential to be linked physically in vivo. Given their temporally distinct localization patterns to actin patches, it is likely that the complexes formed among NPFs change composition throughout patch development. This paints a complex picture of NPF coordination in vivo, and future deciphering of these events will likely require sophisticated microscopy approaches and biochemical tests in purified mixtures and/or cell-free extracts.
Abp1 performs a unique function as an NPF in vivo:
The initial identification and characterization of Abp1 as an NPF (Goode et al. 2001) left two key questions open concerning its function: (1) Why does Abp1 bind to actin filaments and not monomers? and (2) Why does Abp1 have two acidic (A) motifs and tightly associate with Arp2/3 complex through gel filtration, yet have only weak nucleation-promoting activity compared to other NPFs? This latter point took on particular meaning after purified full-length Las17 was shown to have extremely potent and strong nucleation-promoting activity, over 2 orders of magnitude stronger than that of Abp1 (Rodal et al. 2003). The data presented here offer new insights into these questions surrounding Abp1 function.
Collectively, our data raise the possibility that Abp1 suppresses the activity of other NPFs. The primary observation leading to this model is that abp1Δ suppresses the growth defects of both las17Δ and arp2-1, mutations that impair Arp2/3 complex-mediated actin nucleation. Given that las17Δ creates a background in which cell viability depends on Pan1 and Myo3/Myo5 functions, Abp1 may antagonize one or both of these NPFs. Having two acidic domains may enable Abp1 to compete more effectively with other NPFs for functional association with Arp2/3 complex. This could help recycle stronger NPFs after nucleation events and possibly stabilize filament branch points. Alternatively, this could serve as a competition mechanism that helps balance out NPF activities in a spatially and/or temporally controlled manner. Analogously, it has been shown that cortactin displaces WASp after nucleation has occurred (Uruno et al. 2003) and stabilizes Arp2/3-generated actin filament branch points (Weaver et al. 2002). Abp1 (and by analogy cortactin) also might function to attenuate stronger NPFs in vivo. The lack of an actin monomer-binding domain in Abp1 and cortactin likely explains why these two proteins have weak nucleation-promoting activities to begin with. These properties make them well suited for serving as attenuators of stronger NPFs. Conceptually this is similar to the expression of truncated (dummy) growth factor receptors to temper receptor responses to signals at the cell periphery.
What specific role might Abp1 play in patch development? Our data do not directly address this question but raise possibilities. Given the late arrival of Abp1 and Myo3/Myo5 at patches and our genetic results, it is tempting to speculate that Abp1 might attenuate Myo3/Myo5 function, thereby fine-tuning fission events preceding rapid patch movement inward from the cell cortex. This model is also consistent with ABP1 being essential for endocytosis in specific sla2 mutant backgrounds (Wesp et al. 1997) and SLA2 being required for endocytic scission (Kaksonen et al. 2003; Rodal et al. 2005b).
Contributions of Arp2 to barbed end nucleation and auto-inactivation of Arp2/3 complex:
Our biochemical data on arp2 mutants make three important contributions to our understanding of Arp2/3 complex mechanism: (1) They provide the first experimental evidence for barbed end nucleation by Arp2 and/or Arp3 subunits, (2) they demonstrate the importance of barbed end nucleation by Arp2 in vivo, and (3) they offer insights into the mechanism by which Arp2/3 complex is maintained in an inactive state in the absence of NPFs. All current models for Arp2/3 complex function are predicated on the assumption that the barbed ends of Arp2 and Arp3 nucleate actin polymerization (Kelleher et al. 1995; Robinson et al. 2001; Beltzner and Pollard 2004). However, direct experimental data supporting this mechanism have been lacking. This fundamental model is based primarily on the high degree of sequence homology and structural similarity exhibited among Arp2, Arp3, and actin. Here, we found that two mutations located at the barbed end of Arp2 (arp2-1 and arp2-2) are severely impaired for actin nucleation in vitro, providing direct experimental evidence for barbed end nucleation by Arp2/3 complex. Further, since these mutations cause strong defects in cell growth, actin organization, and endocytosis, this means that barbed end nucleation by Arp2/3 complex is critical in vivo.
The residue mutated in arp2-1 (H330L) resides in a loop located at the barbed end (Figure 2A) that is predicted to contact the second actin subunit of the nucleated actin filament (Beltzner and Pollard 2004). This loop, which is divergent from actin, may provide specialized contacts between Arp2 and actin not found in actin-actin interactions, possibly to help stabilize association of the first actin subunits during nucleation. The residue mutated in arp2-2 (G19D) resides in subdomain I at the barbed end. This residue is not solvent exposed, and therefore, the mutation likely disrupts barbed end nucleation by changing the conformation in subdomain I to weaken surface contacts between Arp2 and actin. Since both alleles (arp2-1 and arp2-2) cause actin organizational defects in vivo and show genetic interactions with NPF mutants, this demonstrates that barbed end nucleation by Arp2/3 complex is critical in vivo. Further, these two alleles of arp2 are suppressed by overexpression of Las17 in vivo (Madania et al. 1999), suggesting that their nucleation defects can be rescued in part by overproducing a strong NPF. This is consistent with our biochemical data showing that elevated concentrations of purified Las17 restore nucleation activity to arp2-1 Arp2/3 complex (Figure 4D).
Our data also provide insights into how Arp2/3 complex is maintained in an inactive state, poised for activation by NPFs. In the crystal structure of Arp2/3 complex (NPFs absent), Arp2 and Arp3 are well separated (Robinson et al. 2001), and when NPFs are added, major structural rearrangements bring Arp2 and Arp3 closer to nucleate actin (Goley et al. 2004; Rodal et al. 2005a). Until such activation, what keeps Arp2 and Arp3 spatially separated? A recent study predicts that the specific sequence of Arp2 at the proposed Arp2-Arp3 interaction surface may be important for this (Beltzner and Pollard 2004). Actin-actin interaction surfaces are optimized to facilitate rapid polymerization; however, this surface on Arp2 is divergent from actin, and possibly repels Arp3, creating the requirement for an NPF to bridge and/or stabilize Arp2-Arp3 association. The two residues mutated in arp2-7 are located in subdomain I (F127S) and subdomain II (F203Y) (Figure 2A). Phe127 is not predicted to make contacts with actin or other subunits of the Arp2/3 complex (Beltzner and Pollard 2004). However, Phe203, which corresponds to Bos taurus Phe205, is located at the pointed end of Arp2 and is predicted to be at the Arp3 interaction surface. Thus, substitution of Phe for Tyr (introducing a hydroxyl group) at this position in Arp2 may disrupt normal intermolecular repulsive forces between Arp2 and Arp3, thereby causing constitutive activation.
Arp2/3 complex activity must be strong and tightly regulated in vivo:
Current models hold that the Arp2/3 complex must be tightly regulated in vivo, such that it remains inactive until the appropriate factors signal to NPFs, which in turn stimulate Arp2/3 complex in the correct spatial and temporal manner. Direct comparisons in actin nucleation assays have shown that purified wild-type S. cerevisiae Arp2/3 complex is more constitutively active than bovine Arp2/3 complex (Rodal et al. 2005a). This could be interpreted to suggest that yeast can tolerate a slightly higher baseline of unregulated Arp2/3 nucleation activity than mammals. Alternatively, yeast may express cellular factors that actively dampen or suppress the baseline Arp2/3 complex nucleation activity. Here, we found that arp2-7 increases the constitutive activity of yeast Arp2/3 complex, which causes defects in actin organization at low temperatures and arrest of cell growth at high temperatures. Recently, we described a mutant allele (arc35-5) of a different subunit p35/ARPC2 that is also partially constitutively active in vitro (Rodal et al. 2005a) and has defects in actin organization in vivo. Here we show that combining these two mutations in yeast is lethal, suggesting that there is a threshold of unregulated Arp2/3 complex activity above which cell viability is lost. Similarly, yeast can tolerate single mutations that each partially impair actin nucleation (e.g., arp2-1, arp2-2, arc35-6, las17Δ, myo3Δmyo5Δ, and pan1-101), but most pairwise combinations of these alleles are lethal (Figure 1A and Table 2). Together, these observations demonstrate that the nucleation activity of Arp2/3 complex must be sufficiently potent in vivo, but also properly silenced in the absence of an activator.
Acknowledgments
We are extremely grateful to Avital Rodal for suggesting the use of arp2 alleles to uncouple Arp2/3 regulation and for thoughtful advice throughout this work. We also thank Anya Goodman, Adam Martin, Omar Quintero-Monzon, Avital Rodal, David Shackelford, and Barbara Winsor for generously providing reagents and Heath Balcer, Fred Chang, Wei Guo, Avital Rodal, and Isabelle Sagot for helpful comments on the manuscript. This work was supported by a grant to B.G. from the National Institute of Health (GM63691).
References
- Beltzner, C. C., and T. D. Pollard, 2004. Identification of functionally important residues of Arp2/3 complex by analysis of homology models from diverse species. J. Mol. Biol. 336: 551–565. [DOI] [PubMed] [Google Scholar]
- Duncan, M. C., M. J. Cope, B. L. Goode, B. Wendland and D. G. Drubin, 2001. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 3: 687–690. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., B. M. Klebl, A. H. Tong, B. A. Webb, T. Leeuw et al., 2000. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley, E. D., S. E. Rodenbusch, A. C. Martin and M. D. Welch, 2004. Critical conformational changes in the Arp2/3 complex are induced by nucleotide and nucleation promoting factor. Mol. Cell 16: 269–279. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., 2002. Purification of yeast actin and actin-associated proteins. Methods Enzymol. 351: 433–441. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., and A. A. Rodal, 2001. Modular complexes that regulate actin assembly in budding yeast. Curr. Opin. Microbiol 4: 703–712. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., A. A. Rodal, G. Barnes and D. G. Drubin, 2001. Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J. Cell Biol. 153: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay, C. W., H. Dewar, D. T. Warren, R. Costa, N. Satish et al., 2003. An interaction between Sla1p and Sla2p plays a role in regulating actin dynamics and endocytosis in budding yeast. J. Cell Sci. 116: 2551–2564. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and R. Fink, 1991. Guide to Yeast Genetics and Molecular Biology (Methods in Enzymology, Vol. 194). Academic Press, San Diego. [PubMed]
- Higgs, H. N., L. Blanchoin and T. D. Pollard, 1999. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry 38: 15212–15222. [DOI] [PubMed] [Google Scholar]
- Huang, S., Y. Q. An, J. M. McDowell, E. C. McKinney and R. B. Meagher, 1996. The Arabidopsis thaliana ACT4/ACT12 actin gene subclass is strongly expressed throughout pollen development. Plant J. 10: 189–202. [DOI] [PubMed] [Google Scholar]
- Huckaba, T. M., A. C. Gay, L. F. Pantalena, H. C. Yang and L. A. Pon, 2004. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 167: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. W., 2002. Vacuolar proteases and proteolytic artifacts in Saccharomyces cerevisiae. Methods Enzymol. 351: 127–150. [DOI] [PubMed] [Google Scholar]
- Jonsdottir, G. A., and R. Li, 2004. Dynamics of yeast Myosin I: evidence for a possible role in scission of endocytic vesicles. Curr. Biol. 14: 1604–1609. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., Y. Sun and D. G. Drubin, 2003. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115: 475–487. [DOI] [PubMed] [Google Scholar]
- Kelleher, J. F., S. J. Atkinson and T. D. Pollard, 1995. Sequences, structural models, and cellular localization of the actin-related proteins Arp2 and Arp3 from Acanthamoeba. J. Cell Biol. 131: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D. R., J. R. Kuhn, A. L. Tichy and T. D. Pollard, 2003. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161: 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T., A. Shevchenko and R. Li, 2000. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller, J. P., G. Henry and D. M. Helfman, 1992. Identification of act2, an essential gene in the fission yeast Schizosaccharomyces pombe that encodes a protein related to actin. Proc. Natl. Acad. Sci. USA 89: 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R., 1997. Bee1, a yeast protein with homology to Wiscott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J. Cell Biol. 136: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lila, T., and D. G. Drubin, 1997. Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol. Biol. Cell 8: 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Machesky, L. M., S. J. Atkinson, C. Ampe, J. Vandekerckhove and T. D. Pollard, 1994. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J. Cell Biol. 127: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madania, A., P. Dumoulin, S. Grava, H. Kitamoto, C. Scharer-Brodbeck et al., 1999. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell 10: 3521–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, V., A. Madania, R. P. Martin and B. Winsor, 1996. The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J. Cell Biol. 134: 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F., C. Egile, T. Lipkin and R. Li, 2004. ARPC1/Arc40 mediates the interaction of the actin-related protein 2 and 3 complex with Wiskott-Aldrich syndrome protein family activators. J. Biol. Chem. 279: 54629–54636. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D., 1984. Polymerization of ADP-actin. J. Cell Biol. 99: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, T. D., 1986. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 103: 2747–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Monzon, O., A. A. Rodal, B. Strokopytov, S. C. Almo and B. L. Goode, 2005. Structural and functional dissection of the Abp1 ADFH actin-binding domain reveals versatile in vivo adapter functions. Mol. Biol. Cell 16: 3128–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, R. C., K. Turbedsky, D. A. Kaiser, J. B. Marchand, H. N. Higgs et al., 2001. Crystal structure of Arp2/3 complex. Science 294: 1679–1684. [DOI] [PubMed] [Google Scholar]
- Rodal, A. A., M. Duncan and D. Drubin, 2002. Purification of glutathione S-transferase fusion proteins from yeast. Methods Enzymol. 351: 168–172. [DOI] [PubMed] [Google Scholar]
- Rodal, A. A., A. L. Manning, B. L. Goode and D. G. Drubin, 2003. Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr. Biol. 13: 1000–1008. [DOI] [PubMed] [Google Scholar]
- Rodal, A. A., O. Sokolova, D. B. Robins, K. M. Daugherty, S. Hippenmeyer et al., 2005. a Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat. Struct. Mol. Biol. 12: 26–31. [DOI] [PubMed] [Google Scholar]
- Rodal, A. A., L. Kozubowski, B. L. Goode, D. G. Drubin and J. H. Hartwig, 2005. b Actin and septin ultrastructures at the budding yeast cell cortex. Mol. Biol. Cell 16: 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob, E., and R. P. Martin, 1992. New yeast actin-like gene required late in the cell cycle. Nature 355: 179–182. [DOI] [PubMed] [Google Scholar]
- Tang, H. Y., J. Xu and M. Cai, 2000. Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell Biol. 20: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima, J., J. Y. Toshima, A. C. Martin and D. G. Drubin, 2005. Phosphoregulation of Arp2/3 dependent actin assembly during receptor-mediated endocytosis. Nat. Cell Biol. 7: 246–254. [DOI] [PubMed] [Google Scholar]
- Uruno, T., J. Liu, P. Zhang, Y. Fan, C. Egile et al., 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3: 259–266. [DOI] [PubMed] [Google Scholar]
- Uruno, T., J. Liu, Y. Li, N. Smith and X. Zhan, 2003. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and cortactin during branched actin filament network formation. J. Biol. Chem. 278: 26086–26093. [DOI] [PubMed] [Google Scholar]
- Wallar, B. J., and A. S. Alberts, 2003. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13: 435–446. [DOI] [PubMed] [Google Scholar]
- Weaver, A. M., A. V. Karginov, A. W. Kinley, S. A. Weed, Y. Li et al., 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11: 370–374. [DOI] [PubMed] [Google Scholar]
- Weaver, A. M., J. E. Heuser, A. V. Karginov, W. L. Lee, J. T. Parsons et al., 2002. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr. Biol. 12: 1270–1278. [DOI] [PubMed] [Google Scholar]
- Welch, M. D., and R. D. Mullins, 2002. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 18: 247–288. [DOI] [PubMed] [Google Scholar]
- Wesp, A., L. Hicke, J. Palecek, R. Lombardi, T. Aust et al., 1997. End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 8: 2291–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, D. C., E. Y. Choe and R. Li, 1999. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA 96: 7288–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., J. B. Moseley, I. Sagot, F. Poy, D. Pellman et al., 2004. Crystal structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell 116: 711–723. [DOI] [PubMed] [Google Scholar]