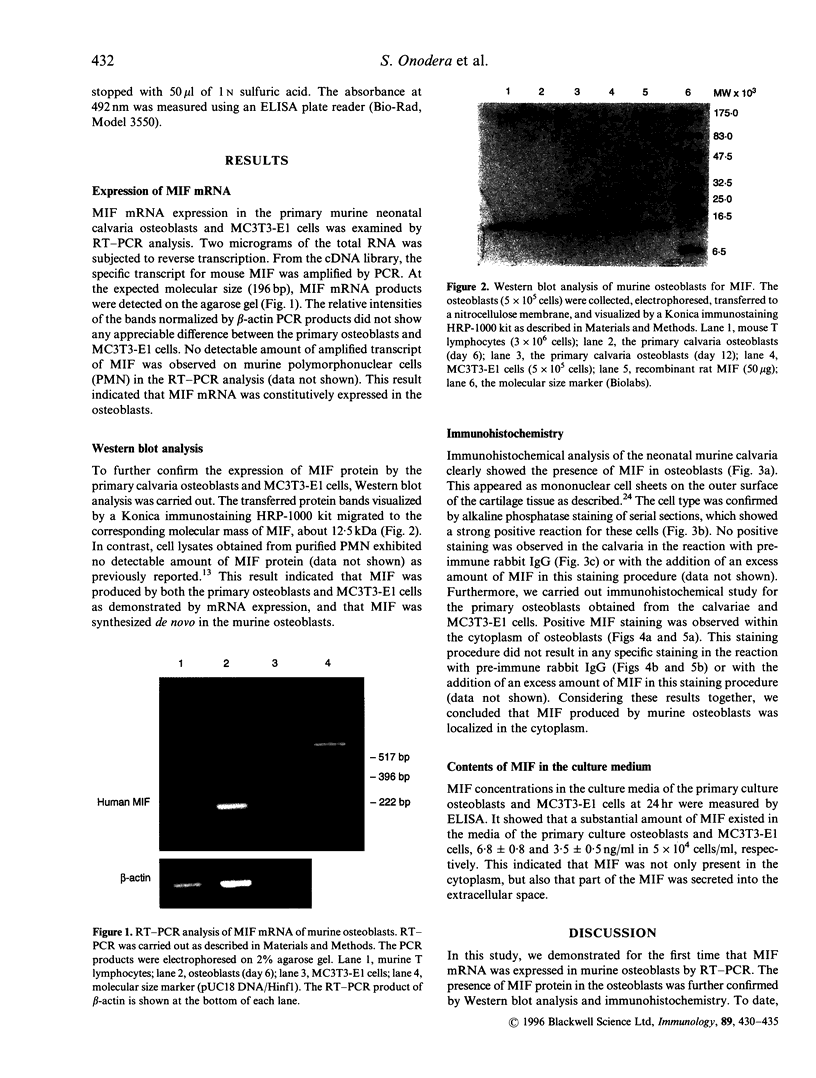
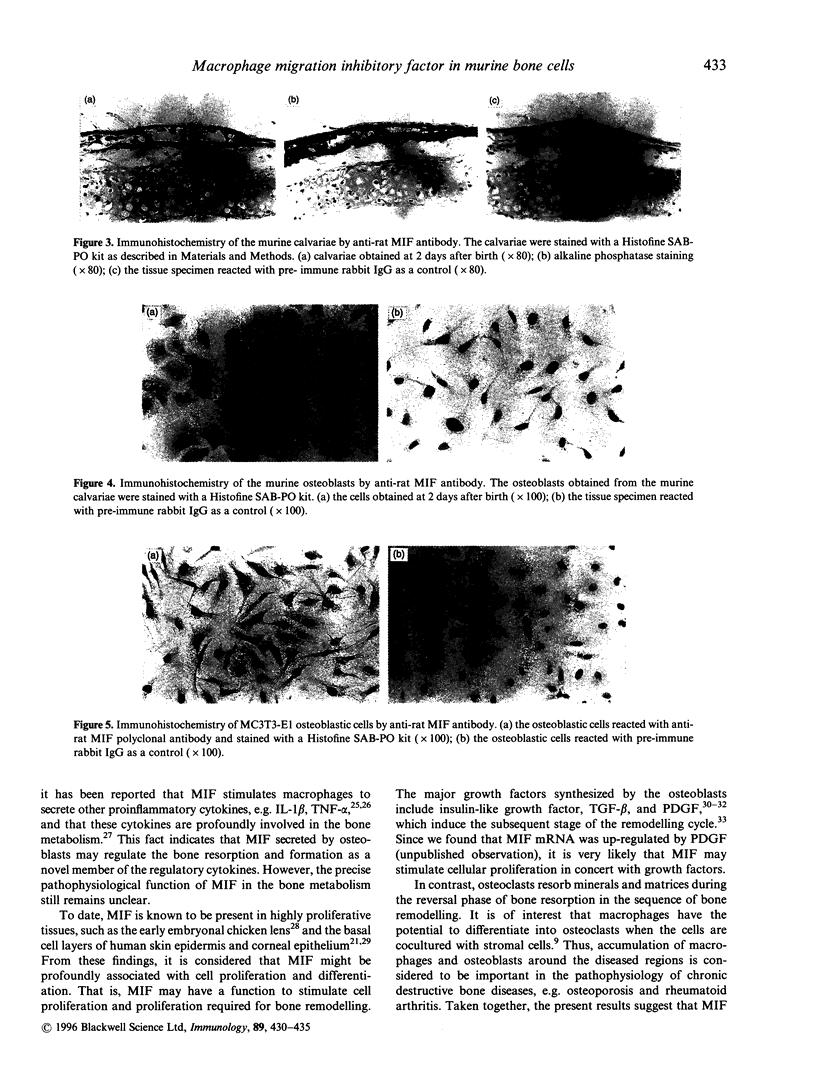
Abstract
Bone resorption and formation are dynamic processes that occur in both normal and injured bone tissues. Regulation of these processes is mediated at the local level by cytokines and growth factors. Macrophage migration inhibitory factor (MIF) is one of the proinflammatory cytokines that activates macrophages and regulates production of other cytokines, such as tumour necrosis factor-alpha and interleukin-1. We here demonstrate, by reverse transcription-polymerase chain reaction, high expression of MIF mRNA in murine osteoblasts obtained from mouse neonatal calvariae and murine osteoblastic MC3T3-E1 cells. The presence of MIF protein in the osteoblasts was confirmed by Western blot analysis using anti-rat MIF antibody. Moreover, the immunohistochemical study revealed that MIF was localized largely in the cytoplasm. The pathophysiological function of MIF remains undefined; however, the present results suggest that MIF takes part in the osseous metabolism as well as in immunological events.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron J. E. Osteocyte types in the developing mouse calvarium. Calcif Tissue Res. 1973;12(4):259–279. doi: 10.1007/BF02013740. [DOI] [PubMed] [Google Scholar]
- Beresford J. N., Gallagher J. A., Gowen M., Couch M., Poser J., Wood D. D., Russell R. G. The effects of monocyte-conditioned medium and interleukin 1 on the synthesis of collagenous and non-collagenous proteins by mouse bone and human bone cells in vitro. Biochim Biophys Acta. 1984 Sep 7;801(1):58–65. doi: 10.1016/0304-4165(84)90212-5. [DOI] [PubMed] [Google Scholar]
- Bernhagen J., Mitchell R. A., Calandra T., Voelter W., Cerami A., Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994 Nov 29;33(47):14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Bolander M. E. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992 Jun;200(2):165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995 Sep 7;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Mitchell R. A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994 Jun 1;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. Effect of glucocorticoids on type I collagen synthesis, alkaline phosphatase activity, and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983 Mar;112(3):931–939. doi: 10.1210/endo-112-3-931. [DOI] [PubMed] [Google Scholar]
- Centrella M., Massagué J., Canalis E. Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology. 1986 Nov;119(5):2306–2312. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Göthlin G. Electron microscopic observations on fracture repair in the rat. Acta Pathol Microbiol Scand A. 1973 Jul;81(4):507–522. doi: 10.1111/j.1699-0463.1973.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Hahn T. J., Westbrook S. L., Halstead L. R. Cortisol modulation of osteoblast metabolic activity in cultured neonatal rat bone. Endocrinology. 1984 May;114(5):1864–1870. doi: 10.1210/endo-114-5-1864. [DOI] [PubMed] [Google Scholar]
- Kanatani M., Sugimoto T., Kano J., Fukase M., Fujita T. Effect of 17 beta-estradiol on the proliferation of osteoblastic MC3T3-E1 cells via human monocytes. Biochem Biophys Res Commun. 1991 Aug 15;178(3):866–870. doi: 10.1016/0006-291x(91)90971-9. [DOI] [PubMed] [Google Scholar]
- Marcus R. Normal and abnormal bone remodeling in man. Annu Rev Med. 1987;38:129–141. doi: 10.1146/annurev.me.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- Matsuda A., Tagawa Y., Matsuda H., Nishihira J. Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett. 1996 May 6;385(3):225–228. doi: 10.1016/0014-5793(96)00386-9. [DOI] [PubMed] [Google Scholar]
- Minkin C., Shapiro I. M. Osteoclasts, mononuclear phagocytes, and physiological bone resorption. Calcif Tissue Int. 1986 Dec;39(6):357–359. doi: 10.1007/BF02555171. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihira J., Kuriyama T., Nishino H., Ishibashi T., Sakai M., Nishi S. Purification and characterization of human macrophage migration inhibitory factor: evidence for specific binding to glutathione and formation of subunit structure. Biochem Mol Biol Int. 1993 Dec;31(5):841–850. [PubMed] [Google Scholar]
- Nishihira J., Kuriyama T., Sakai M., Nishi S., Ohki S., Hikichi K. The structure and physicochemical properties of rat liver macrophage migration inhibitory factor. Biochim Biophys Acta. 1995 Feb 22;1247(1):159–162. doi: 10.1016/0167-4838(94)00215-3. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., D'Souza S. M., Mundy G. R. Effects of transforming growth factor-beta on osteoblastic osteosarcoma cells. Endocrinology. 1987 Jul;121(1):212–218. doi: 10.1210/endo-121-1-212. [DOI] [PubMed] [Google Scholar]
- Pozzi L. A., Weiser W. Y. Human recombinant migration inhibitory factor activates human macrophages to kill tumor cells. Cell Immunol. 1992 Dec;145(2):372–379. doi: 10.1016/0008-8749(92)90339-q. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988 Mar 31;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Werb Z. Macrophage-derived growth factors. Curr Top Microbiol Immunol. 1992;181:87–140. doi: 10.1007/978-3-642-77377-8_4. [DOI] [PubMed] [Google Scholar]
- Rifas L., Cheng S. L., Shen V., Peck W. A. Monokines produced by macrophages stimulate the growth of osteoblasts. Connect Tissue Res. 1989;23(2-3):163–178. doi: 10.3109/03008208909002416. [DOI] [PubMed] [Google Scholar]
- Sakai M., Nishihira J., Hibiya Y., Koyama Y., Nishi S. Glutathione binding rat liver 13k protein is the homologue of the macrophage migration inhibitory factor. Biochem Mol Biol Int. 1994 Jun;33(3):439–446. [PubMed] [Google Scholar]
- Sakurai A., Satomi N., Haranaka K. Tumour necrosis factor and the lysosomal enzymes of macrophages or macrophage-like cell line. Cancer Immunol Immunother. 1985;20(1):6–10. doi: 10.1007/BF00199766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ohkawara A., Nishihira J., Sakamoto W. Identification of macrophage migration inhibitory factor (MIF) in human skin and its immmunohistochemical localization. FEBS Lett. 1996 Mar 4;381(3):199–202. doi: 10.1016/0014-5793(96)00120-2. [DOI] [PubMed] [Google Scholar]
- Sugimoto H., Suzuki M., Nakagawa A., Tanaka I., Fujinaga M., Nishihira J. Crystallization of rat liver macrophage migration inhibitory factor for MAD analysis. J Struct Biol. 1995 Nov-Dec;115(3):331–334. doi: 10.1006/jsbi.1995.1057. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Murata E., Tanaka I., Nishihira J., Sakai M. Crystallization and a preliminary X-ray diffraction study of macrophage migration inhibitory factor from human lymphocytes. J Mol Biol. 1994 Jan 21;235(3):1141–1143. doi: 10.1006/jmbi.1994.1063. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sugimoto H., Nakagawa A., Tanaka I., Nishihira J., Sakai M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996 Mar;3(3):259–266. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W. Y., Temple P. A., Witek-Giannotti J. S., Remold H. G., Clark S. C., David J. R. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Shaughnessy M. P., Lee D. C., Hodin J., Zelenka P. S. A macrophage migration inhibitory factor is expressed in the differentiating cells of the eye lens. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1272–1275. doi: 10.1073/pnas.90.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. L. Basal activities and hormone responsiveness of osteoclast-like and osteoblast-like bone cells are regulated by glucocorticoids. J Biol Chem. 1979 Jul 25;254(14):6337–6340. [PubMed] [Google Scholar]
- Wong G., Cohn D. V. Separation of parathyroid hormone and calcitonin-sensitive cells from non-responsive bone cells. Nature. 1974 Dec 20;252(5485):713–715. doi: 10.1038/252713a0. [DOI] [PubMed] [Google Scholar]