Abstract
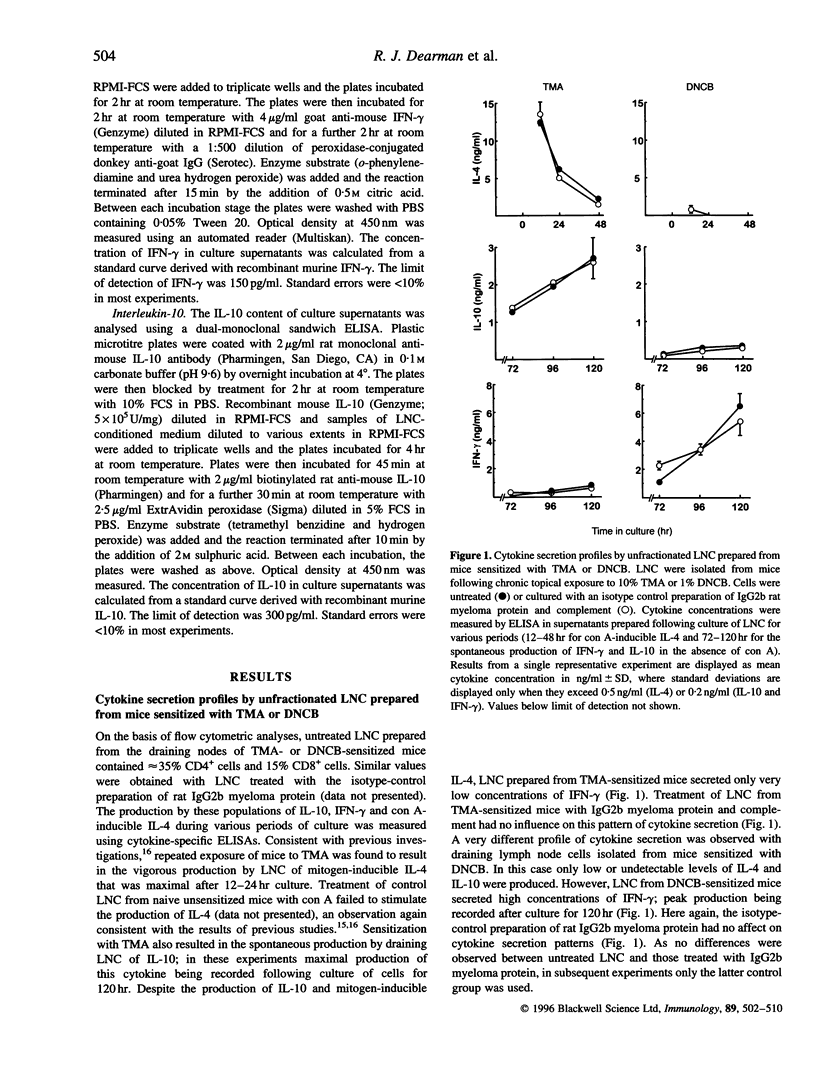
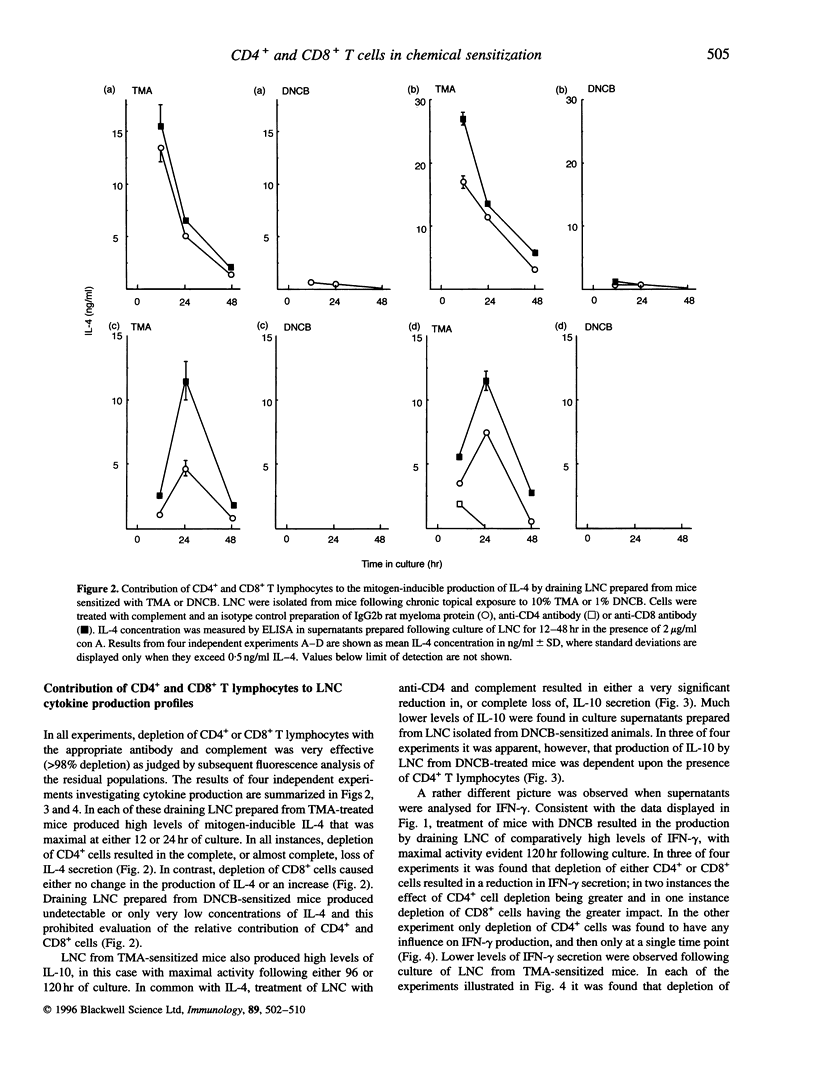
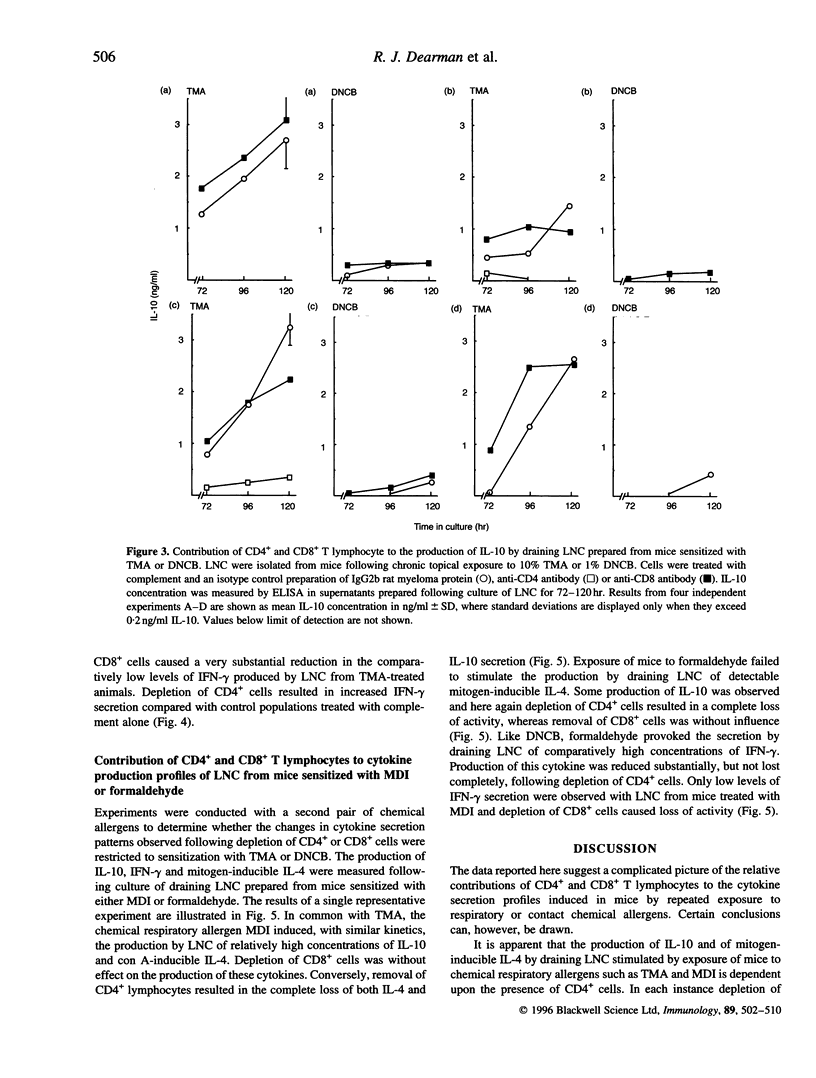
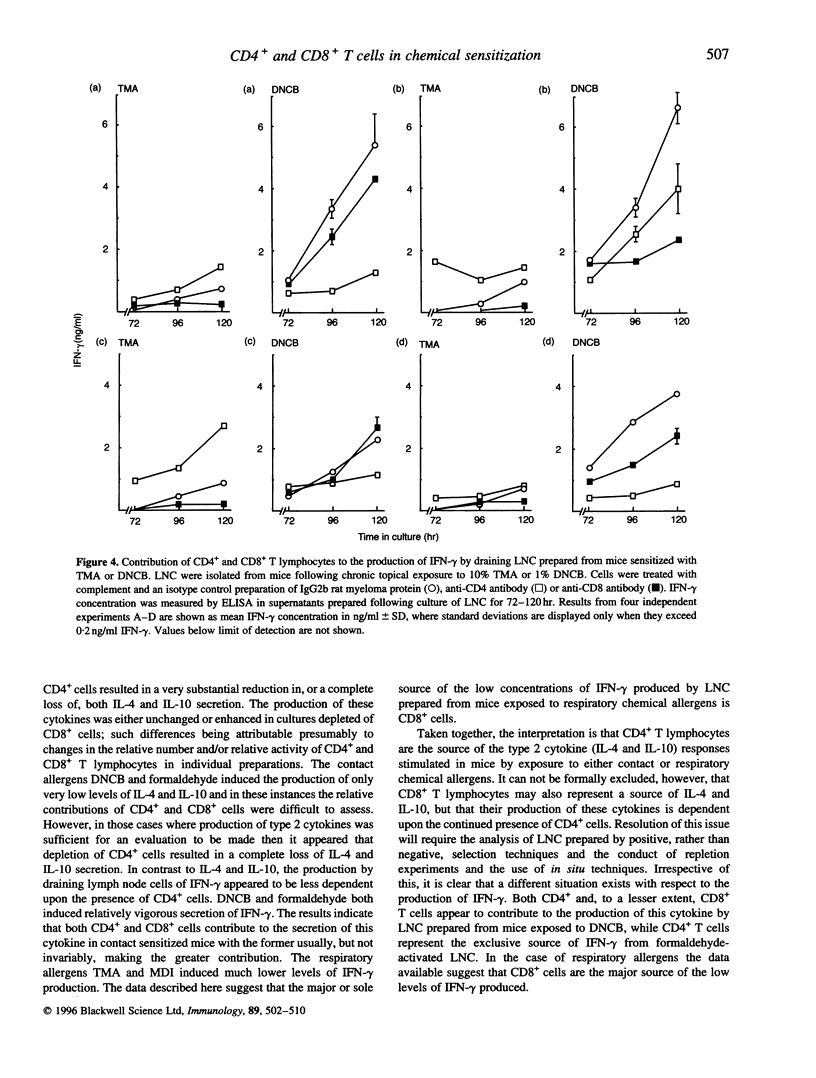
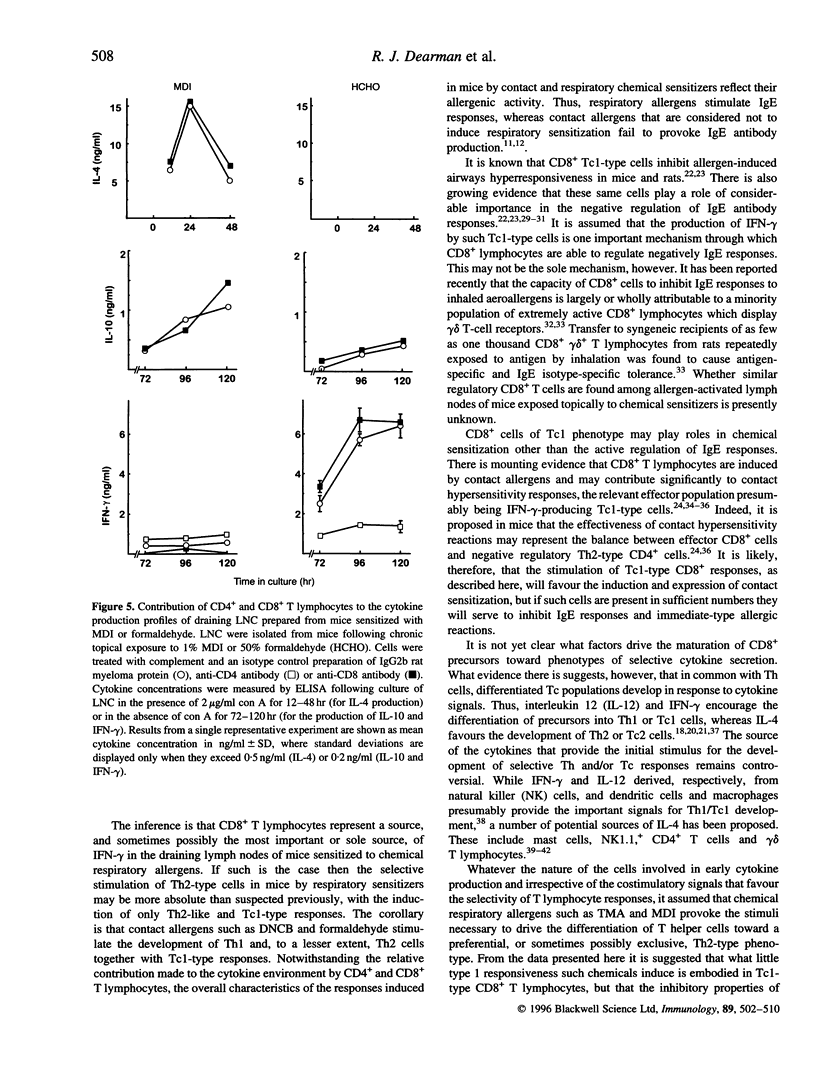
Chemical allergens of different types, those that cause in humans allergic contact dermatitis or occupational asthma induce in mice divergent immune responses characteristic, respectively, of T-helper 1 (Th1)- and Th2-type cell activation. Such responses are associated with the development of different cytokine secretion patterns by draining lymph node cells (LNC), such that contact allergens stimulate vigorous interferon-gamma (IFN-gamma) production, but little secretion of the Th2 cytokines interleukin-4 and interleukin-10 (IL-4 and IL-10), whereas the converse pattern is provoked by respiratory allergens. Using selective depletion with antibody and complement we have here examined the relative contribution of CD4+ and CD8+ T lymphocytes to the cytokine secretion patterns of draining LNC isolated from mice sensitized to chemical allergens. Mice received repeated topical applications of respiratory allergens, trimellitic anhydride (TMA) or diphenylmethane diisocyanate (MDI), or of contact allergens 2,4-dinitrochlorobenzene (DNCB) or formaldehyde. Thirteen days following the initiation of exposure the production by draining LNC of IL-10, IFN-gamma and mitogen (concanavalin A)-inducible IL-4 was measured by enzyme-linked immunosorbent assay (ELISA) after various periods of culture. It was found that the high levels of IL-4 and IL-10 secretion stimulated by TMA or MDI, and the lower levels of these cytokines induced by DNCB or formaldehyde, were in all cases dependent upon the presence of CD4- cells. In contrast, the comparatively high concentrations of IFN-gamma observed following exposure to contact allergens were found to be derived from CD4+ cells, and in the case of DNCB from CD8+ cells also. The low levels of IFN-gamma induced by treatment with TMA or MDI were associated largely or wholly with CD8+ cells. These data indicate that the type 2 cytokine responses induced to different extents by both contact and respiratory chemical allergens are almost exclusively a function of CD4+ cells, but that IFN-gamma is produced by either CD4+ cells in the case of contact allergens or largely by CD8+ cells in the case of chemical respiratory allergens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bour H., Peyron E., Gaucherand M., Garrigue J. L., Desvignes C., Kaiserlian D., Revillard J. P., Nicolas J. F. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995 Nov;25(11):3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Hudak S., Jackson J., Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989 Jul 21;245(4915):308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- Croft M., Carter L., Swain S. L., Dutton R. W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994 Nov 1;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Basketter D. A., Kimber I. Differential cytokine production following chronic exposure of mice to chemical respiratory and contact allergens. Immunology. 1995 Dec;86(4):545–550. [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Differential stimulation of immune function by respiratory and contact chemical allergens. Immunology. 1991 Apr;72(4):563–570. [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Divergent immune responses to respiratory and contact chemical allergens: antibody elicited by phthalic anhydride and oxazolone. Clin Exp Allergy. 1992 Feb;22(2):241–250. doi: 10.1111/j.1365-2222.1992.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Mitchell J. A., Basketter D. A., Kimber I. Differential ability of occupational chemical contact and respiratory allergens to cause immediate and delayed dermal hypersensitivity reactions in mice. Int Arch Allergy Immunol. 1992;97(4):315–321. doi: 10.1159/000236139. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Ramdin L. S., Basketter D. A., Kimber I. Inducible interleukin-4-secreting cells provoked in mice during chemical sensitization. Immunology. 1994 Apr;81(4):551–557. [PMC free article] [PubMed] [Google Scholar]
- Fehr B. S., Takashima A., Matsue H., Gerometta J. S., Bergstresser P. R., Cruz P. D., Jr Contact sensitization induces proliferation of heterogeneous populations of hapten-specific T cells. Exp Dermatol. 1994 Aug;3(4):189–197. doi: 10.1111/j.1600-0625.1994.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Ferrick D. A., Schrenzel M. D., Mulvania T., Hsieh B., Ferlin W. G., Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995 Jan 19;373(6511):255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
- Gocinski B. L., Tigelaar R. E. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990 Jun 1;144(11):4121–4128. [PubMed] [Google Scholar]
- Hilton J., Dearman R. J., Basketter D. A., Scholes E. W., Kimber I. Experimental assessment of the sensitizing properties of formaldehyde. Food Chem Toxicol. 1996 Jun;34(6):571–578. doi: 10.1016/0278-6915(96)00012-9. [DOI] [PubMed] [Google Scholar]
- Holliday M. R., Coleman J. W., Dearman R. J., Kimber I. Induction of mast cell sensitization by chemical allergens: a comparative study. J Appl Toxicol. 1993 Mar-Apr;13(2):137–142. doi: 10.1002/jat.2550130211. [DOI] [PubMed] [Google Scholar]
- Iwamoto I., Nakajima H., Endo H., Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993 Feb 1;177(2):573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Diaz-Sanchez D. The role of CD8+ T cells in the regulation of IgE. Clin Exp Allergy. 1993 Jun;23(6):466–470. doi: 10.1111/j.1365-2222.1993.tb03232.x. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Noble A., Holmes B. J., Diaz-Sanchez D. Immune regulation: a new role for the CD8+ T cell. Immunol Today. 1994 Mar;15(3):107–110. doi: 10.1016/0167-5699(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Keskinen H., Tupasela O., Tiikkainen U., Nordman H. Experiences of specific IgE in asthma due to diisocyanates. Clin Allergy. 1988 Nov;18(6):597–604. doi: 10.1111/j.1365-2222.1988.tb02911.x. [DOI] [PubMed] [Google Scholar]
- McMenamin C., Holt P. G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993 Sep 1;178(3):889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin C., McKersey M., Kühnlein P., Hünig T., Holt P. G. Gamma delta T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995 May 1;154(9):4390–4394. [PubMed] [Google Scholar]
- McMenamin C., Pimm C., McKersey M., Holt P. G. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994 Sep 23;265(5180):1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996 Mar;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Noble A., Macary P. A., Kemeny D. M. IFN-gamma and IL-4 regulate the growth and differentiation of CD8+ T cells into subpopulations with distinct cytokine profiles. J Immunol. 1995 Sep 15;155(6):2928–2937. [PubMed] [Google Scholar]
- Renz H., Lack G., Saloga J., Schwinzer R., Bradley K., Loader J., Kupfer A., Larsen G. L., Gelfand E. W. Inhibition of IgE production and normalization of airways responsiveness by sensitized CD8 T cells in a mouse model of allergen-induced sensitization. J Immunol. 1994 Jan 1;152(1):351–360. [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Sad S., Marcotte R., Mosmann T. R. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995 Mar;2(3):271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Underwood S. L., Kemeny D. M., Lee T. H., Raeburn D., Karlsson J. A. IgE production, antigen-induced airway inflammation and airway hyperreactivity in the brown Norway rat: the effects of ricin. Immunology. 1995 Jun;85(2):256–261. [PMC free article] [PubMed] [Google Scholar]
- Xu H., DiIulio N. A., Fairchild R. L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996 Mar 1;183(3):1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Hu-Li J., Paul W. E. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Watson C., Hu-Li J., Paul W. E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995 Dec 15;270(5243):1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]


