Abstract
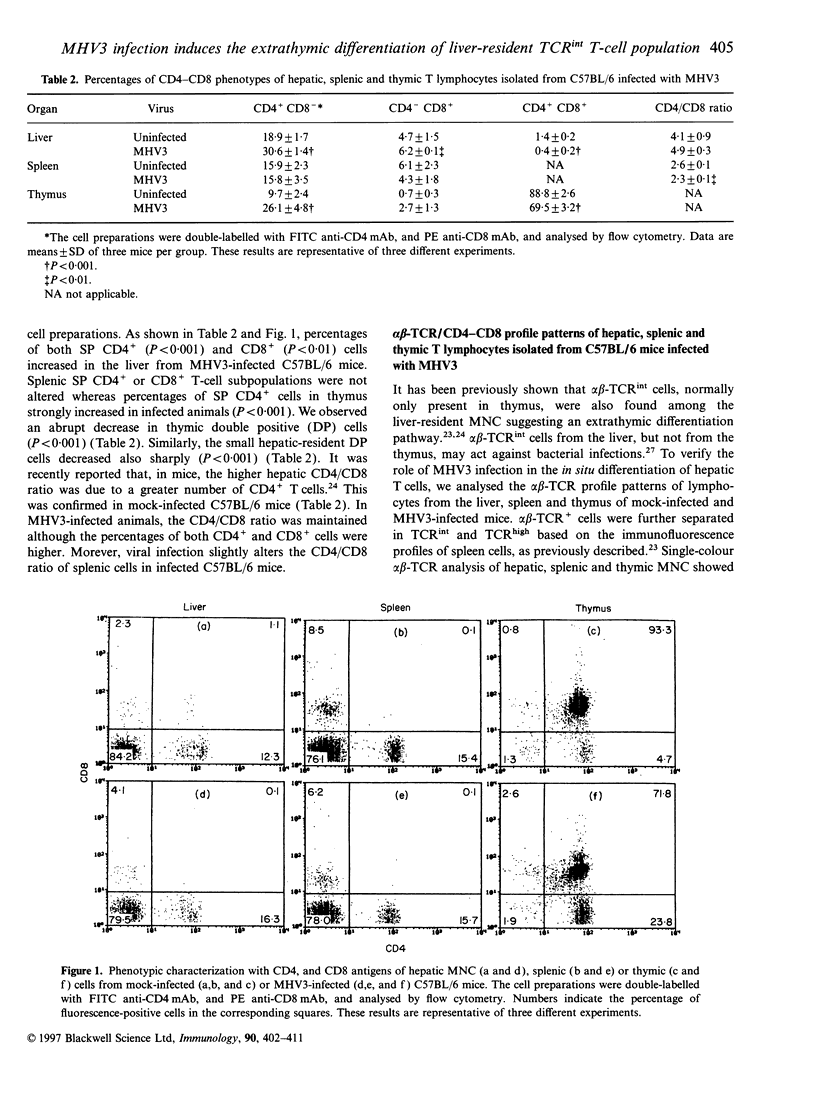
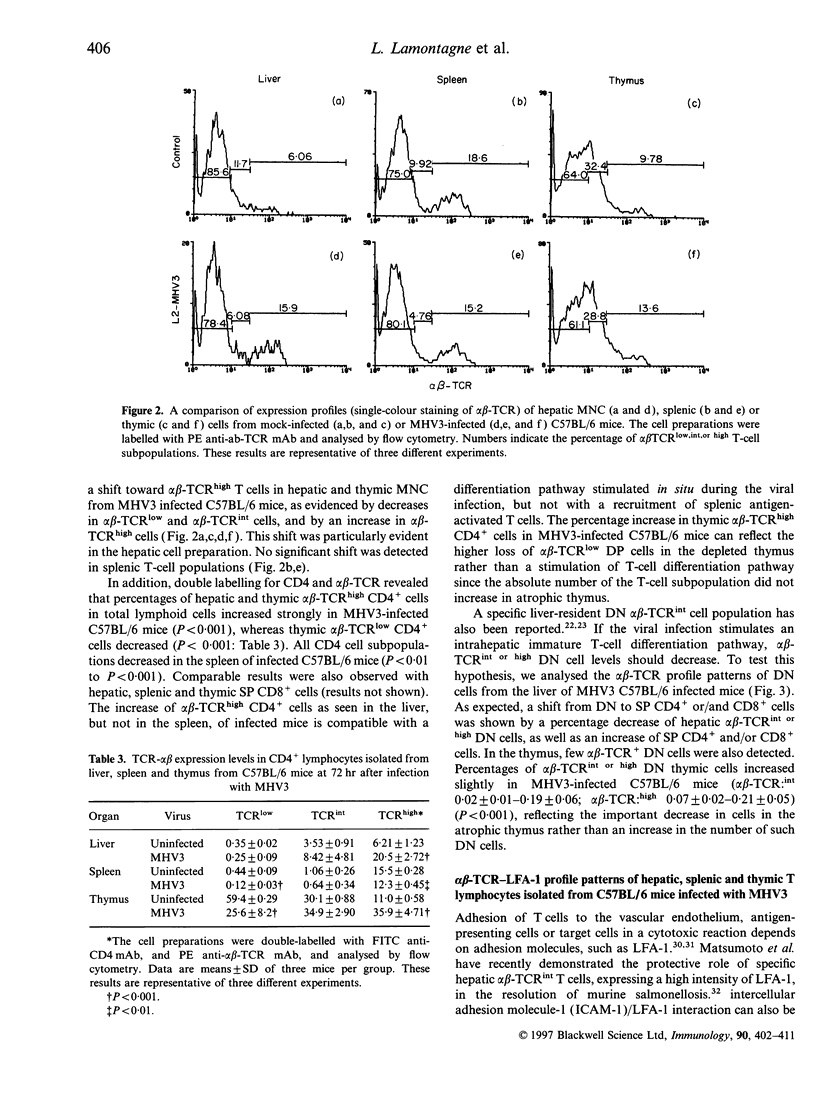
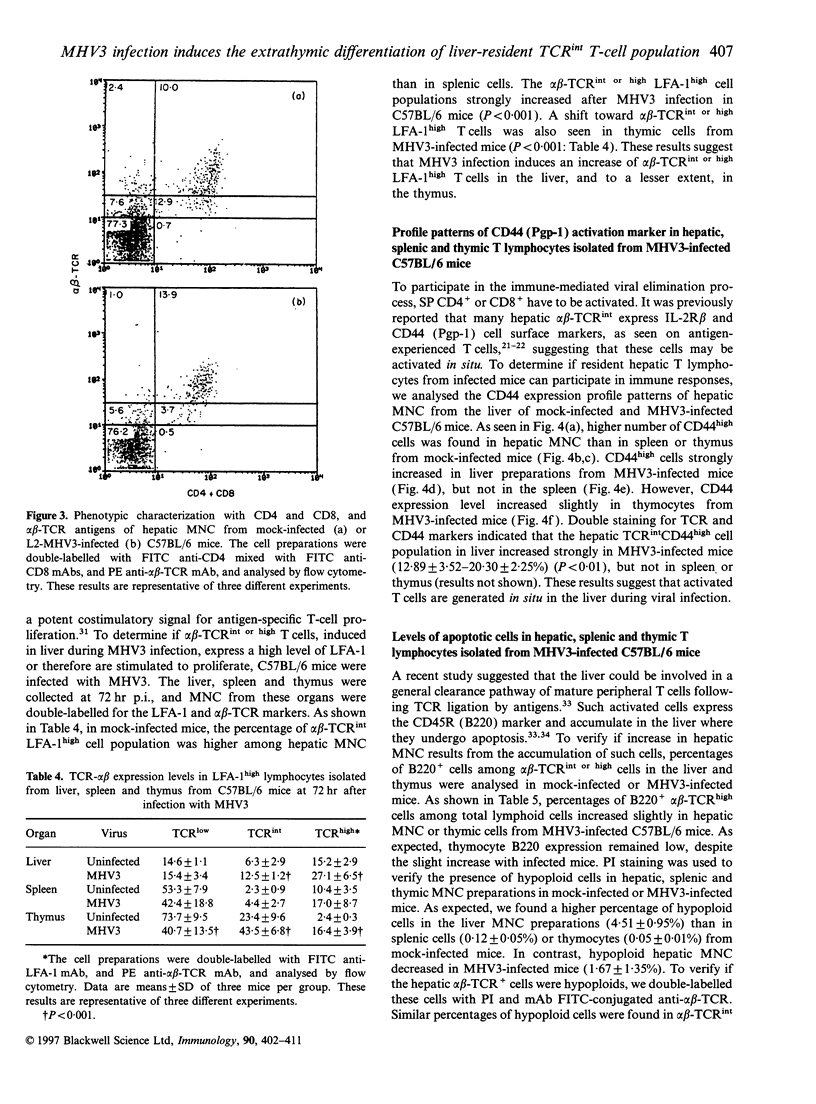
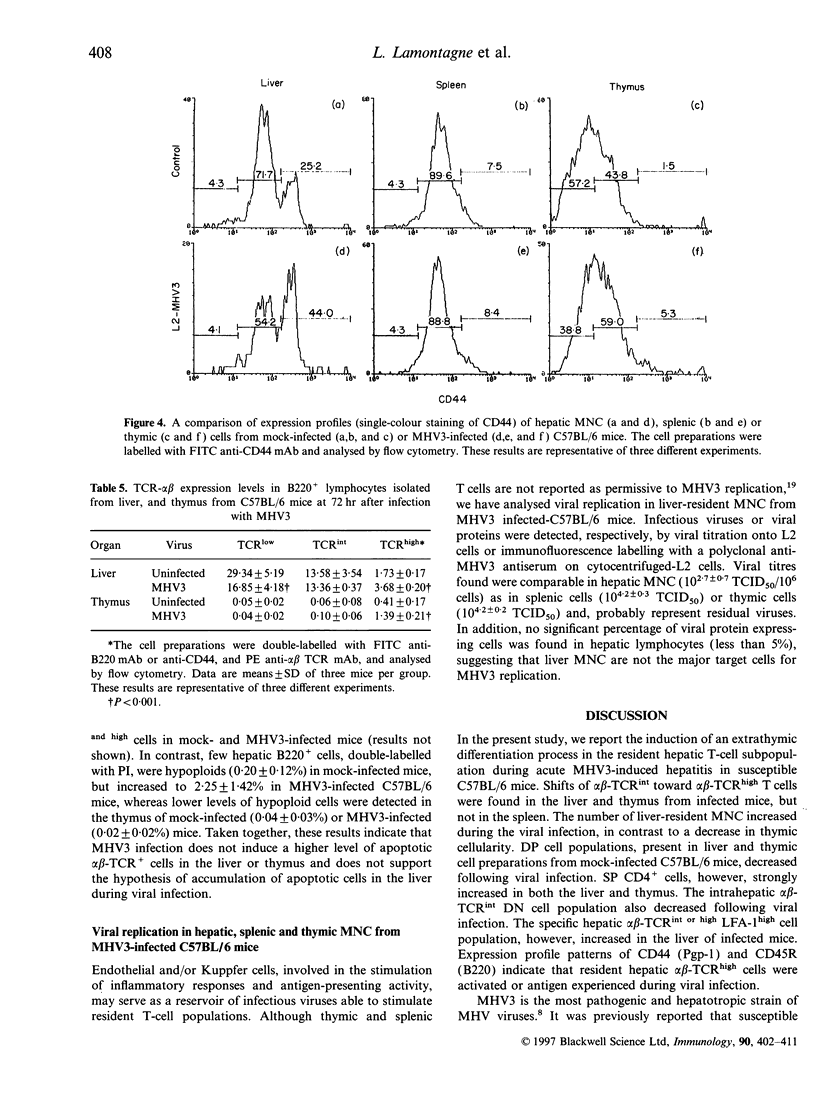
Mouse hepatitis virus type 3 (MHV3), a coronavirus, is an excellent model for the study of thymic and extrathymic T-cell subpopulation disorders induced during viral hepatitis. It was recently reported that, in addition to the intrathymic T-cell differentiation pathway, an extrathymic differentiation pathway of alpha beta-T-cell receptor (TCR) T lymphocytes exists in the liver, and becomes important under pathological situations such as autoimmune diseases, malignancies or hepatic bacterial infections. In the present study, we compared the phenotypes of resident hepatic, splenic or thymic T-cell subpopulations during the acute viral hepatitis induced by HMV3 in susceptible C57BL/6 mice. The number of liver-resident mononuclear cells (MNC) increased during the viral infection, while cellularity decreased. Single positive (SP) CD4+ cells strongly increased in both the liver and thymus, while double positive (DP) (CD4+ CD8+) cells, present in the liver and thymus of mock-infected mice, decreased in C57BL/6 mice during the viral infection. A shift of alpha beta-TCRintermediate T cells toward alpha beta-TCRhigh was evidenced in the liver and thymus of infected mice, but not in the spleen. The few alpha beta-TCRint double negative (DN) (CD4-CD8-) cells also decreased following viral infection. alpha beta-TCRint or high lymphocytes expressing high levels of leucocyte function antigen-1 (LFA-1) increased in the liver of MHV3-infected mice. In addition, liver-resident T cells expressed strongly the CD44 (Pgp-1) activation marker, suggesting that they were either activated or antigen experienced during the viral infection. No significant change in T-cell subpopulations was detected in the spleen, suggesting that MHV3 infection could induce an early in situ differentiation of resident hepatic T cells rather than a recruitment of lymphocytes from peripheral lymphoid organs.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Ohteki T., Seki S., Koyamada N., Yoshikai Y., Masuda T., Rikiishi H., Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991 Aug 1;174(2):417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter H., Baechi T., Haller O. Adult mouse hepatocytes in primary monolayer culture express genetic resistance to mouse hepatitis virus type 3. J Immunol. 1982 Sep;129(3):1275–1281. [PubMed] [Google Scholar]
- Barthold S. W. Host age and genotypic effects on enterotropic mouse hepatitis virus infection. Lab Anim Sci. 1987 Feb;37(1):36–40. [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Gorczynski R., Cruz B., Fingerote R., Skamene E., Perlman S., Leibowitz J., Fung L., Flowers M., Levy G. A Th1 cell line (3E9.1) from resistant A/J mice inhibits induction of macrophage procoagulant activity in vitro and protects against MHV-3 mortality in vivo. Immunology. 1994 Nov;83(3):353–361. [PMC free article] [PubMed] [Google Scholar]
- Conrad P., Rothman B. L., Kelley K. A., Blue M. L. Mechanism of peripheral T cell activation by coengagement of CD44 and CD2. J Immunol. 1992 Sep 15;149(6):1833–1839. [PubMed] [Google Scholar]
- Cook-Mills J. M., Munshi H. G., Perlman R. L., Chambers D. A. Mouse hepatitis virus infection suppresses modulation of mouse spleen T-cell activation. Immunology. 1992 Mar;75(3):542–545. [PMC free article] [PubMed] [Google Scholar]
- Dindzans V. J., Skamene E., Levy G. A. Susceptibility/resistance to mouse hepatitis virus strain 3 and macrophage procoagulant activity are genetically linked and controlled by two non-H-2-linked genes. J Immunol. 1986 Oct 1;137(7):2355–2360. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Décimo D., Boespflug O., Meunier-Rotival M., Hadchouel M., Tardieu M. Genetic restriction of murine hepatitis virus type 3 expression in liver and brain: comparative study in BALB/c and C3H mice by immunochemistry and hybridization in situ. Arch Virol. 1993;130(3-4):269–277. doi: 10.1007/BF01309659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J. S., Kruisbeek A. M. The role of LFA-1/ICAM-1 interactions during murine T lymphocyte development. J Immunol. 1991 Nov 1;147(9):2852–2859. [PubMed] [Google Scholar]
- Fung-Leung W. P., Kündig T. M., Zinkernagel R. M., Mak T. W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991 Dec 1;174(6):1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvy B. A., Telford W. G., King L. E., Fraker P. J. Glucocorticoids and irradiation-induced apoptosis in normal murine bone marrow B-lineage lymphocytes as determined by flow cytometry. Immunology. 1993 Jun;79(2):270–277. [PMC free article] [PubMed] [Google Scholar]
- Huang L., Soldevila G., Leeker M., Flavell R., Crispe I. N. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994 Dec;1(9):741–749. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Lamontagne L. Mouse hepatitis virus 3 pathogenicity expressed by a lytic viral infection in bone marrow 14.8+ mu+ B lymphocyte subpopulations. J Immunol. 1989 Dec 1;143(11):3722–3730. [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Lamontagne L., Descoteaux J. P., Jolicoeur P. Mouse hepatitis virus 3 replication in T and B lymphocytes correlate with viral pathogenicity. J Immunol. 1989 Jun 15;142(12):4458–4465. [PubMed] [Google Scholar]
- Lamontagne L., Jolicoeur P. Mouse hepatitis virus 3-thymic cell interactions correlating with viral pathogenicity. J Immunol. 1991 May 1;146(9):3152–3159. [PubMed] [Google Scholar]
- Le Prevost C., Levy-Leblond E., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. Role of humoral and cell-mediated immunity in resistance mechanisms. J Immunol. 1975 Jan;114(1 Pt 1):221–225. [PubMed] [Google Scholar]
- Levy G. A., Leibowitz J. L., Edgington T. S. Induction of monocyte procoagulant activity by murine hepatitis virus type 3 parallels disease susceptibility in mice. J Exp Med. 1981 Oct 1;154(4):1150–1163. doi: 10.1084/jem.154.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B., Vasseur F., Penit C. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2'-deoxyuridine-pulse-labeled thymocytes. Correlation with T cell receptor expression. J Immunol. 1993 Nov 1;151(9):4574–4582. [PubMed] [Google Scholar]
- Lucas B., Vasseur F., Penit C. Production, selection, and maturation of thymocytes with high surface density of TCR. J Immunol. 1994 Jul 1;153(1):53–62. [PubMed] [Google Scholar]
- Martin J. P., Chen W., Koehren F., Pereira C. A. The virulence of mouse hepatitis virus 3, as evidenced by permissivity of cultured hepatic cells toward escape mutants. Res Virol. 1994 Sep-Oct;145(5):297–302. doi: 10.1016/S0923-2516(07)80034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., Okuyama R., Seki S., Abo T., Sugiura K., Kusumi A., Ohmori T., Watanabe H., Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992 Sep 1;149(5):1562–1570. [PubMed] [Google Scholar]
- Pham B. N., Martinot-Peignoux M., Mosnier J. F., Njapoum C., Marcellin P., Bougy F., Degott C., Erlinger S., Cohen J. H., Degos F. CD4+/CD8+ ratio of liver-derived lymphocytes is related to viraemia and not to hepatitis C virus genotypes in chronic hepatitis C. Clin Exp Immunol. 1995 Nov;102(2):320–327. doi: 10.1111/j.1365-2249.1995.tb03784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham B. N., Mosnier J. F., Walker F., Njapoum C., Bougy F., Degott C., Erlinger S., Cohen J. H., Degos F. Flow cytometry CD4+/CD8+ ratio of liver-derived lymphocytes correlates with viral replication in chronic hepatitis B. Clin Exp Immunol. 1994 Sep;97(3):403–410. doi: 10.1111/j.1365-2249.1994.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M., Chung S. W., Mosmann T., Leibowitz J. L., Gorczynski R. M., Levy G. A. Resistance of naive mice to murine hepatitis virus strain 3 requires development of a Th1, but not a Th2, response, whereas pre-existing antibody partially protects against primary infection. J Immunol. 1996 May 1;156(9):3342–3349. [PubMed] [Google Scholar]
- Prévost C. L., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. III. Clinical and virologic observation of a persistent viral infection. J Immunol. 1975 Sep;115(3):640–643. [PubMed] [Google Scholar]
- Raff M. C., Owen J. J. Thymus-derived lymphocytes: their distribution and role in the development of peripheral lymphoid tissues of the mouse. Eur J Immunol. 1971 Jan;1(1):27–30. doi: 10.1002/eji.1830010105. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Sprent J. Lifespans of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993 Jun;5(3):433–438. doi: 10.1016/0952-7915(93)90065-z. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama Y., Tiruppathi C., offakidevi K., Andersen T. T., Fenton J. W., 2nd, Malik A. B. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J Cell Biol. 1992 Nov;119(4):935–944. doi: 10.1083/jcb.119.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990 Jul;12(1):59–65. doi: 10.1002/hep.1840120110. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Ohtsuka K., Kimura M., Ikarashi Y., Ohmori K., Kusumi A., Ohteki T., Seki S., Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992 Feb 5;146(2):145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]


