Abstract
Rheumatoid factors (RF) are the characteristic autoantibodies found in patients with rheumatoid arthritis. They recognize epitopes in the Fc region of immunoglobulin G (IgG) and are often of the IgM isotype. In order to analyse the nature of RF-Fc interactions, we have crystallized a complex between the Fab fragment of a human monoclonal IgM rheumatoid factor (RF-AN) and the Fc fragment of human IgG4. The stoichiometry of the complex within the crystals was found to be 2:1 Fab:Fc. The crystals diffracted X-rays to 0.3 nm resolution, and the space group was C2, with cell dimensions a = 16.03 nm, b = 8.19 nm, c = 6.42 nm, beta = 98.3 degrees. We have also determined the sequence of the variable region of the RF-AN light chain, not hitherto reported. This belongs to the V lambda III-a subgroup and is closely related to the germline gene Humlv318, from which it differs in three amino acid residues. This is the first reported crystallized complex between a human autoantibody and its autoantigen.
Full text
PDF


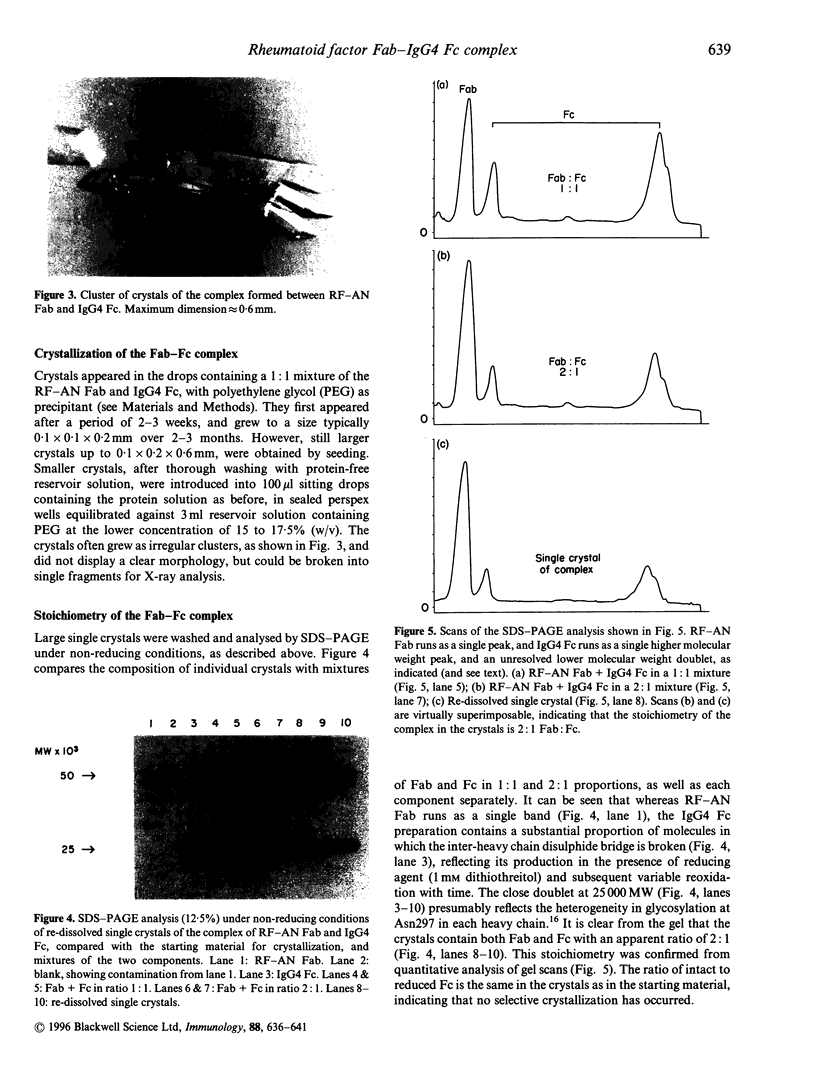


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. M., Plater-Zyberk C., Mageed R. A., Jefferis R., Maini R. N. Analysis of immunoglobulins secreted by hybridomas derived from rheumatoid synovia. Clin Exp Immunol. 1990 Jun;80(3):366–372. doi: 10.1111/j.1365-2249.1990.tb03294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister W. P., Huber A. H., Bjorkman P. J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994 Nov 24;372(6504):379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Ermel R. W., Kenny T. P., Chen P. P., Robbins D. L. Molecular analysis of rheumatoid factors derived from rheumatoid synovium suggests an antigen-driven response in inflamed joints. Arthritis Rheum. 1993 Mar;36(3):380–388. doi: 10.1002/art.1780360314. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Murphy C., Weiss D. T., Solomon A. Serologic and chemical differentiation of human lambda III light chain variable regions. J Immunol. 1991 May 1;146(9):3091–3096. [PubMed] [Google Scholar]
- Harindranath N., Goldfarb I. S., Ikematsu H., Burastero S. E., Wilder R. L., Notkins A. L., Casali P. Complete sequence of the genes encoding the VH and VL regions of low- and high-affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int Immunol. 1991 Sep;3(9):865–875. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R., Lund J., Mizutani H., Nakagawa H., Kawazoe Y., Arata Y., Takahashi N. A comparative study of the N-linked oligosaccharide structures of human IgG subclass proteins. Biochem J. 1990 Jun 15;268(3):529–537. doi: 10.1042/bj2680529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R., Nik Jaafar M. I., Steinitz M. Immunogenic and antigenic epitopes of immunoglobulins. VIII. A human monoclonal rheumatoid factor having specificity for a discontinuous epitope determined by histidine/arginine interchange as residue 435 of immunoglobulin G. Immunol Lett. 1984;7(4):191–194. doi: 10.1016/0165-2478(84)90042-7. [DOI] [PubMed] [Google Scholar]
- Mageed R. A., Carson D. A., Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins. XXIII. Idiotypy and molecular specificity of human rheumatoid factors: analysis of cross-reactive idiotype of rheumatoid factor paraproteins from the Wa idiotype group in relation to their IgG subclass specificity. Scand J Immunol. 1988 Aug;28(2):233–240. doi: 10.1111/j.1365-3083.1988.tb02436.x. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Natvig J. B., Thompson K., Randen I., Steinitz M., Taussig M., Beale D., Barker P., Sletten K., Waalen K., Førre O. Partial amino acid sequence analysis and variable subgroup determination (VH and VL) of a monoclonal rheumatoid factor derived from a rheumatoid arthritis patient. Scand J Rheumatol Suppl. 1988;75:127–132. doi: 10.3109/03009748809096753. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988 Apr 30;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Pascual V., Randen I., Thompson K., Sioud M., Forre O., Natvig J., Capra J. D. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. Further evidence that some autoantibodies are unmutated copies of germ line genes. J Clin Invest. 1990 Oct;86(4):1320–1328. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Victor K., Randen I., Thompson K., Steinitz M., Førre O., Fu S. M., Natvig J. B., Capra J. D. Nucleotide sequence analysis of rheumatoid factors and polyreactive antibodies derived from patients with rheumatoid arthritis reveals diverse use of VH and VL gene segments and extensive variability in CDR-3. Scand J Immunol. 1992 Aug;36(2):349–362. doi: 10.1111/j.1365-3083.1992.tb03108.x. [DOI] [PubMed] [Google Scholar]
- Peterson C., Malone C. C., Williams R. C., Jr Rheumatoid-factor-reactive sites on CH3 established by overlapping 7-mer peptide epitope analysis. Mol Immunol. 1995 Jan;32(1):57–75. doi: 10.1016/0161-5890(94)00122-h. [DOI] [PubMed] [Google Scholar]
- Rudd P. M., Leatherbarrow R. J., Rademacher T. W., Dwek R. A. Diversification of the IgG molecule by oligosaccharides. Mol Immunol. 1991 Dec;28(12):1369–1378. doi: 10.1016/0161-5890(91)90039-m. [DOI] [PubMed] [Google Scholar]
- Sauer-Eriksson A. E., Kleywegt G. J., Uhlén M., Jones T. A. Crystal structure of the C2 fragment of streptococcal protein G in complex with the Fc domain of human IgG. Structure. 1995 Mar 15;3(3):265–278. doi: 10.1016/s0969-2126(01)00157-5. [DOI] [PubMed] [Google Scholar]
- Sohi M. K., Sutton B. J., Corper A. L., Wan T., Maini R. N., Brown C., Rijnders T., Beale D., Feinstein A., Humphreys A. S. Crystallization and preliminary X-ray analysis of the Fab fragment of a human monoclonal IgM rheumatoid factor (2A2). J Mol Biol. 1994 Oct 7;242(5):706–708. doi: 10.1006/jmbi.1994.1620. [DOI] [PubMed] [Google Scholar]
- Solomon A., Weiss D. T. Serologically defined V region subgroups of human lambda light chains. J Immunol. 1987 Aug 1;139(3):824–830. [PubMed] [Google Scholar]
- Steinitz M., Izak G., Cohen S., Ehrenfeld M., Flechner I. Continuous production of monoclonal rheumatoid factor by EBV-transformed lymphocytes. Nature. 1980 Oct 2;287(5781):443–445. doi: 10.1038/287443a0. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Tamir S. Human monoclonal autoimmune antibody produced in vitro: rheumatoid factor generated by Epstein-Barr virus-transformed cell line. Eur J Immunol. 1982 Feb;12(2):126–133. doi: 10.1002/eji.1830120206. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- van Zeben D., Rook G. A., Hazes J. M., Zwinderman A. H., Zhang Y., Ghelani S., Rademacher T. W., Breedveld F. C. Early agalactosylation of IgG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Br J Rheumatol. 1994 Jan;33(1):36–43. doi: 10.1093/rheumatology/33.1.36. [DOI] [PubMed] [Google Scholar]