Abstract
The expanded CD8+ T-lymphocyte population arising in response to viral infection controls the virus but could also prove damaging to the host unless safely removed at the end of the immune response. Apoptosis provides a mechanism whereby this can be achieved, as apoptotic cells are recognized and engulfed by macrophages. Peripheral blood CD8+ T lymphocytes from individuals with acute viral infections were highly susceptible to apoptosis after short-term culture in vitro. This spontaneous cell death could be prevented by interleukin-2 (IL-2) and was related to a decreased expression of Bcl-2 but not Bax or Bcl-XL, additional molecules that promote or prevent apoptosis, respectively, as well as an increase in CD95. After stimulation with anti-CD3 antibody, T cells from these patients also underwent an activation-induced cell death (AICD) that could not be prevented by IL-2. Interestingly, CD8+ T cells from this patient group expressed lower than normal levels of three costimulatory molecules, CD28, CD5 and CD6, suggesting that stimulation in the absence of a second signal is a possible mechanism for the defective reactivation of these cells. Thus multiple mechanisms, including loss of Bcl-2, increased CD95 and loss of costimulatory molecules, place constraints on the survival and reactivation of activated CD8+ T cells after viral infections. This enables immune activation to be controlled and cellular homeostasis to be re-established during resolution of viral diseases in vivo.
Full text
PDF

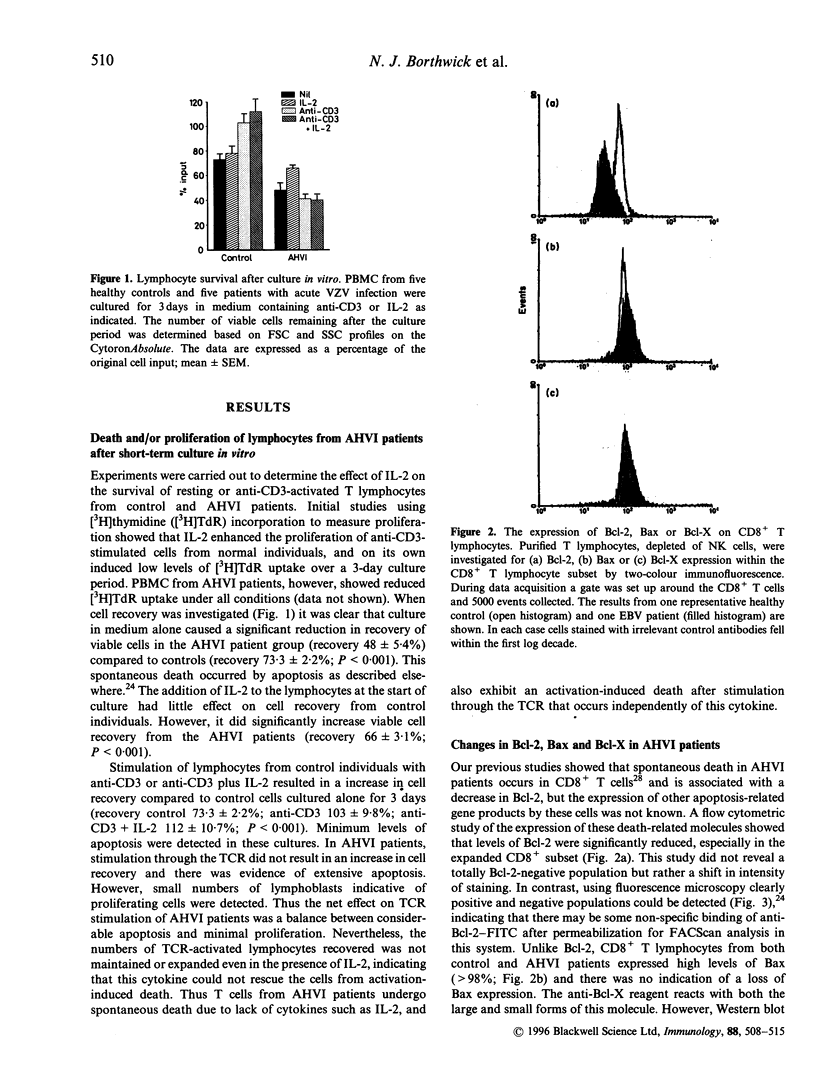
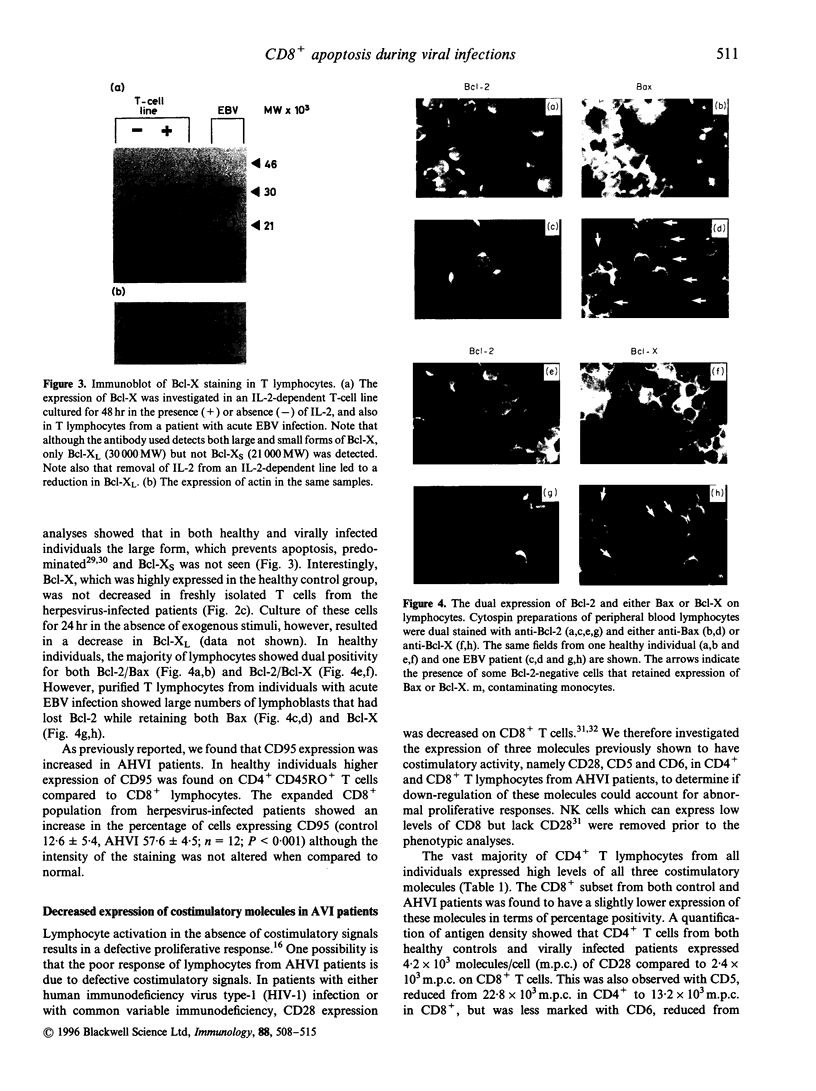
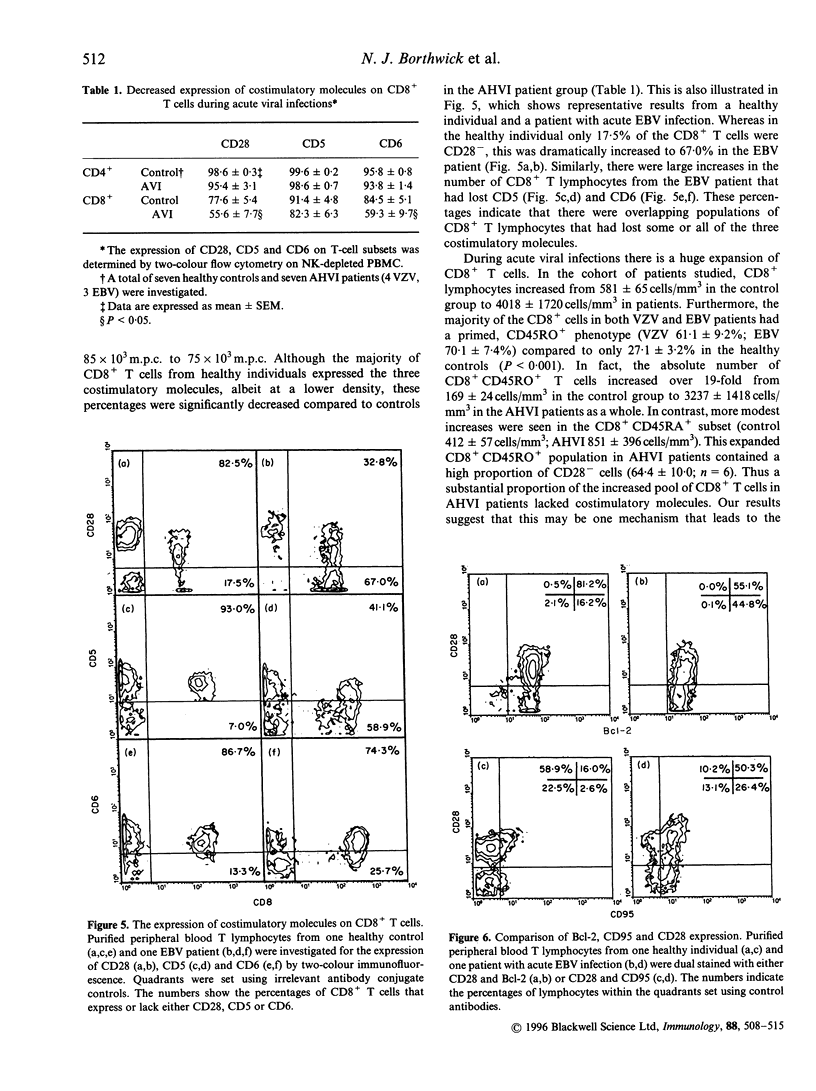



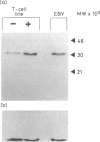
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Borthwick N. J., Wickremasinghe R. G., Panayoitidis P., Pilling D., Bofill M., Krajewski S., Reed J. C., Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996 Feb;26(2):294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Borthwick N., Salmon M., Gombert W., Bofill M., Shamsadeen N., Pilling D., Pett S., Grundy J. E., Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A. N., Salmon M., Savill J., Janossy G. A possible role for bcl-2 in regulating T-cell memory--a 'balancing act' between cell death and survival. Immunol Today. 1993 Nov;14(11):526–532. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Savill J., Gombert W., Bofill M., Borthwick N. J., Whitelaw F., Grundy J., Janossy G., Salmon M. The specific recognition by macrophages of CD8+,CD45RO+ T cells undergoing apoptosis: a mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994 Nov 1;180(5):1943–1947. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill M., Gombert W., Borthwick N. J., Akbar A. N., McLaughlin J. E., Lee C. A., Johnson M. A., Pinching A. J., Janossy G. Presence of CD3+CD8+Bcl-2(low) lymphocytes undergoing apoptosis and activated macrophages in lymph nodes of HIV-1+ patients. Am J Pathol. 1995 Jun;146(6):1542–1555. [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Boise L. H., Minn A. J., Noel P. J., June C. H., Accavitti M. A., Lindsten T., Thompson C. B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995 Jul;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Borthwick N. J., Bofill M., Gombert W. M., Akbar A. N., Medina E., Sagawa K., Lipman M. C., Johnson M. A., Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS. 1994 Apr;8(4):431–441. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Brunner T., Mogil R. J., LaFace D., Yoo N. J., Mahboubi A., Echeverri F., Martin S. J., Force W. R., Lynch D. H., Ware C. F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995 Feb 2;373(6513):441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Azuma M., Okumura K., Lanier L. L., Banchereau J. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med. 1994 Nov 1;180(5):1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J Immunol. 1986 Sep 15;137(6):1816–1821. [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Crawford D. H., Brickell P., Tidman N., McConnell I., Hoffbrand A. V., Janossy G. Increased numbers of cells with suppressor T cell phenotype in the peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol. 1981 Feb;43(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Duvall E., Wyllie A. H., Morris R. G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 1985 Oct;56(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Enssle K. H., Fleischer B. Absence of Epstein-Barr virus-specific, HLA class II-restricted CD4+ cytotoxic T lymphocytes in infectious mononucleosis. Clin Exp Immunol. 1990 Mar;79(3):409–415. doi: 10.1111/j.1365-2249.1990.tb08104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Tsujimoto Y., Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993 Jul 15;151(2):621–627. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- Ju S. T., Panka D. J., Cui H., Ettinger R., el-Khatib M., Sherr D. H., Stanger B. Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995 Feb 2;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Pohl T., Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993 Jul;14(7):338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Wang H. G., Irie S., Fong L., Reed J. C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994 Nov 1;54(21):5501–5507. [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Linsley P. S. Costimulation of T-cell growth. Curr Opin Immunol. 1992 Jun;4(3):265–270. doi: 10.1016/0952-7915(92)90075-p. [DOI] [PubMed] [Google Scholar]
- Maher P., O'Toole C. M., Wreghitt T. G., Spiegelhalter D. J., English T. A. Cytomegalovirus infection in cardiac transplant recipients associated with chronic T cell subset ratio inversion with expansion of a Leu-7+ TS-C+ subset. Clin Exp Immunol. 1985 Dec;62(3):515–524. [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Kasahara Y., Kanegane H., Ohta K., Yokoi T., Yachie A., Taniguchi N. Expression of CD45R0 (UCHL1) by CD4+ and CD8+ T cells as a sign of in vivo activation in infectious mononucleosis. Clin Exp Immunol. 1991 Mar;83(3):447–451. doi: 10.1111/j.1365-2249.1991.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- North M. E., Akbar A. N., Borthwick N., Sagawa K., Funauchi M., Webster A. D., Farrant J. Co-stimulation with anti-CD28 (Kolt-2) enhances DNA synthesis by defective T cells in common variable immunodeficiency. Clin Exp Immunol. 1994 Feb;95(2):204–208. doi: 10.1111/j.1365-2249.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Iwai K., Kasahara Y., Taniguchi N., Krajewski S., Reed J. C., Miyawaki T. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol. 1995 Nov;7(11):1817–1825. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Panayiotidis P., Ganeshaguru K., Jabbar S. A., Hoffbrand A. V. Alpha-interferon (alpha-IFN) protects B-chronic lymphocytic leukaemia cells from apoptotic cell death in vitro. Br J Haematol. 1994 Jan;86(1):169–173. doi: 10.1111/j.1365-2141.1994.tb03269.x. [DOI] [PubMed] [Google Scholar]
- Razvi E. S., Welsh R. M. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993 Oct;67(10):5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Russell J. H., White C. L., Loh D. Y., Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Pilling D., Borthwick N. J., Viner N., Janossy G., Bacon P. A., Akbar A. N. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994 Apr;24(4):892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- Shi Y., Radvanyi L. G., Sharma A., Shaw P., Green D. R., Miller R. G., Mills G. B. CD28-mediated signaling in vivo prevents activation-induced apoptosis in the thymus and alters peripheral lymphocyte homeostasis. J Immunol. 1995 Aug 15;155(4):1829–1837. [PubMed] [Google Scholar]
- Suda T., Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994 Mar 1;179(3):873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swack J. A., Mier J. W., Romain P. L., Hull S. R., Rudd C. E. Biosynthesis and post-translational modification of CD6, a T cell signal-transducing molecule. J Biol Chem. 1991 Apr 15;266(11):7137–7143. [PubMed] [Google Scholar]
- Tan P., Anasetti C., Hansen J. A., Melrose J., Brunvand M., Bradshaw J., Ledbetter J. A., Linsley P. S. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993 Jan 1;177(1):165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testi R., Lanier L. L. Functional expression of CD28 on T cell antigen receptor gamma/delta-bearing T lymphocytes. Eur J Immunol. 1989 Jan;19(1):185–188. doi: 10.1002/eji.1830190129. [DOI] [PubMed] [Google Scholar]
- Tuosto L., Piazza C., Moretti S., Modesti A., Greenlaw R., Lechler R., Lombardi G., Piccolella E. Ligation of either CD2 or CD28 rescues CD4+ T cells from HIV-gp120-induced apoptosis. Eur J Immunol. 1995 Oct;25(10):2917–2922. doi: 10.1002/eji.1830251031. [DOI] [PubMed] [Google Scholar]
- Uehara T., Miyawaki T., Ohta K., Tamaru Y., Yokoi T., Nakamura S., Taniguchi N. Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood. 1992 Jul 15;80(2):452–458. [PubMed] [Google Scholar]