Abstract
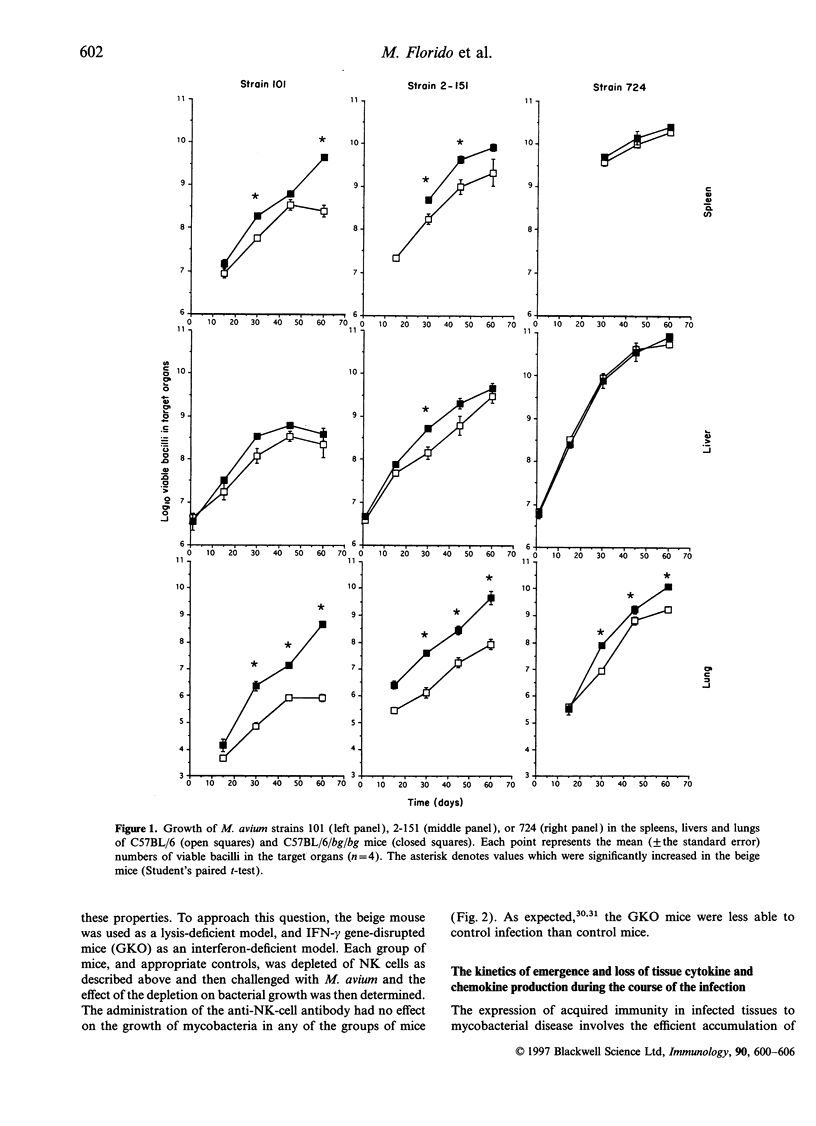
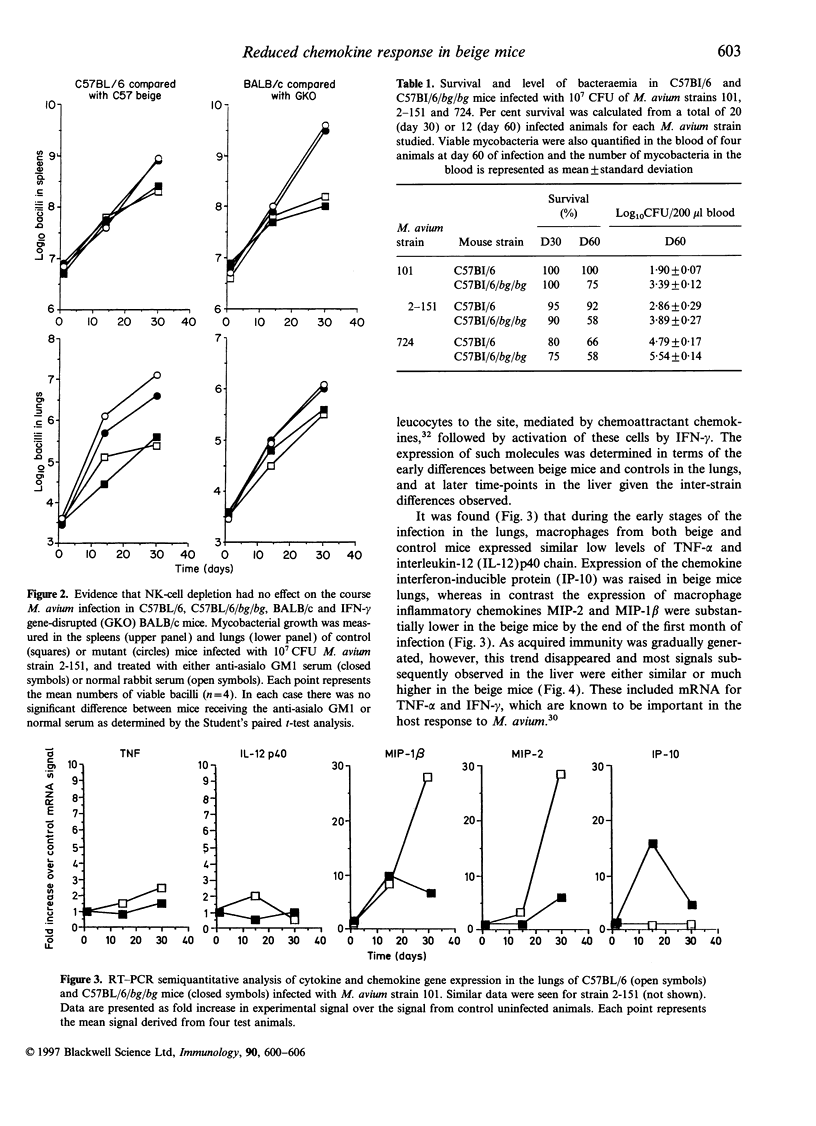
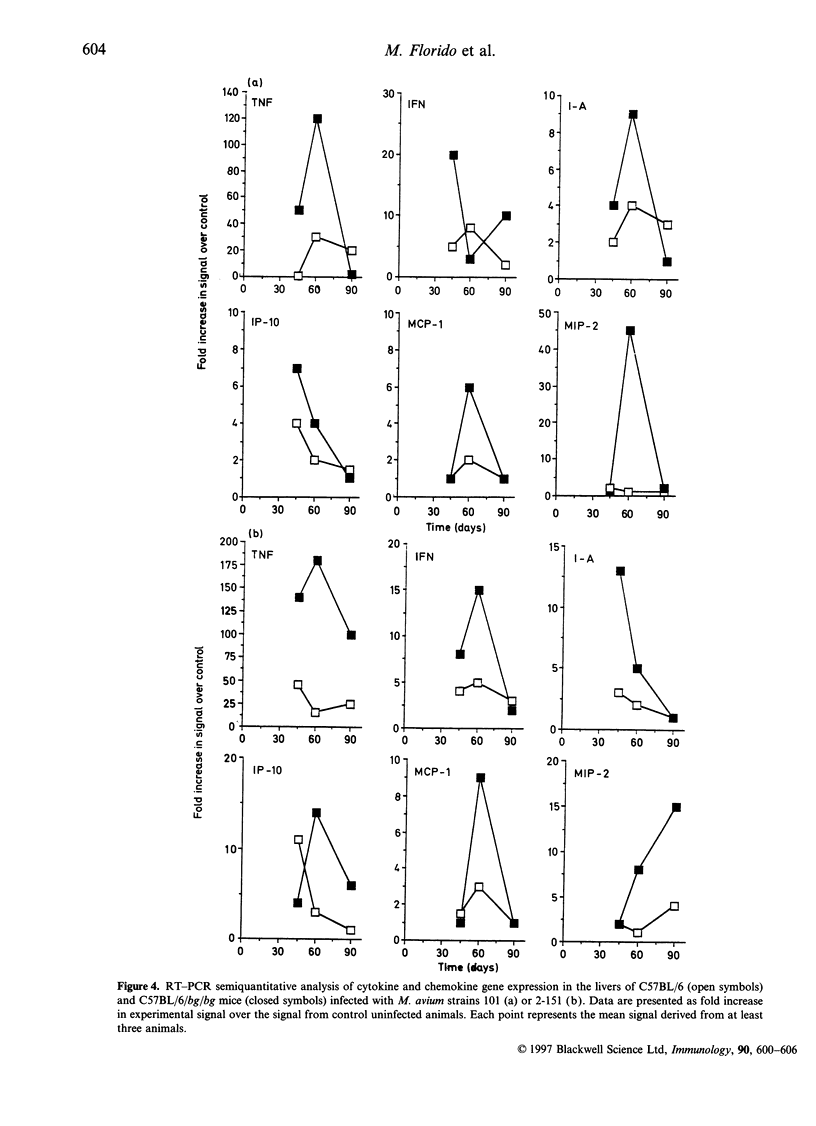
The basis of the increased susceptibility of beige mice to Mycobacterium avium infections is still not clearly understood. In this study we examined the growth of three virulent strains of M. avium in beige mice and normal C57BL/6 controls. Depletion of natural killer (NK) cells by administration of anti-asialo GM1 antisera did not affect the growth of M. avium in any of the groups of animals. Similarly, interferon-gamma (IFN-gamma) gene-disrupted mice were more susceptible to infection than control mice but the growth of M. avium was not further affected by NK-cell depletion. In terms of effector immunity, beige mice showed enhanced expression of IFN-gamma and tumour necrosis factor-alpha (TNF-alpha) when compared with wild-type C57BL/6 mice. In agreement with these results; I-A and interferon-inducible protein (IP-10) expression was also higher in beige mice than in wild-type animals, as was expression of the chemokines macrophage inflammatory protein-2 (MIP-2) and macrophage chemotactic protein (MCP-1) during latter stages of the infection. However, over the first few weeks of the infection, when the susceptibility of the beige mouse lung first becomes evident, MIP-1 beta and MIP-2 chemokine expression in the lungs was lower in beige mice than in wild-type animals. These data indicate, therefore, that the increased susceptibility of beige mice to M. avium infection in the lung is not due to lack of NK-cell activity, nor can it be explained in terms of the effector cytokine response. Instead, the lower early expression of the neutrophil chemoattractants MIP-1 beta and MIP-2 in the lungs of beige mice tends to suggest that the enhanced susceptibility of these mice to M. avium infection may be due in part to defective recruitment of neutrophils or other cells responsive to these specific chemokines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Castro A. G., Gomes S., Pedrosa J., Silva M. T. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995 Sep;63(9):3381–3387. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Castro A. G., Pedrosa J., Silva R. A., Orme I. M., Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994 Sep;62(9):3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R. Interferon-gamma (IFN-gamma) and macrophage inflammatory proteins (MIP)-1 and -2 are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992 Aug;89(2):269–273. doi: 10.1111/j.1365-2249.1992.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Sarmento A., Castro A. G. Tumour necrosis factor-alpha (TNF-alpha) in the host resistance to mycobacteria of distinct virulence. Clin Exp Immunol. 1995 Aug;101(2):308–313. doi: 10.1111/j.1365-2249.1995.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca M. E., Mowat A. M., Parrott D. M. Immunological studies of NK cell-deficient beige mice. II. Analysis of T-lymphocyte functions in beige mice. Immunology. 1989 Jan;66(1):131–137. [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. D., Nguyen Q. A., Tchernev V. T., Ashley J. A., Detter J. C., Blaydes S. M., Brandt S. J., Chotai D., Hodgman C., Solari R. C. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996 Jul 18;382(6588):262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle J. T., McNeil M. R., Chatterjee D., Inamine J. M., Brennan P. J. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993 May 15;268(14):10510–10516. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bertram M. A., Inderlied C. B., Yadegar S., Kolanoski P., Yamada J. K., Young L. S. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986 Jul;154(1):194–195. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- Castro A. G., Minóprio P., Appelberg R. The relative impact of bacterial virulence and host genetic background on cytokine expression during Mycobacterium avium infection of mice. Immunology. 1995 Aug;85(4):556–561. [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Callahan J. E., Griffin J. P., Roberts A. D., Orme I. M. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect Immun. 1995 Sep;63(9):3259–3265. doi: 10.1128/iai.63.9.3259-3265.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynamon M. H., Klemens S. P. Activity of azithromycin against Mycobacterium avium infection in beige mice. Antimicrob Agents Chemother. 1992 Aug;36(8):1611–1613. doi: 10.1128/aac.36.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Modulation of Mycobacterium avium growth in vivo by cytokines: involvement of tumour necrosis factor in resistance to atypical mycobacteria. Clin Exp Immunol. 1991 Mar;83(3):466–471. doi: 10.1111/j.1365-2249.1991.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furney S. K., Roberts A. D., Orme I. M. Effect of rifabutin on disseminated Mycobacterium avium infections in thymectomized, CD4 T-cell-deficient mice. Antimicrob Agents Chemother. 1990 Sep;34(9):1629–1632. doi: 10.1128/aac.34.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R. Beige mouse model for Mycobacterium avium complex disease. Antimicrob Agents Chemother. 1995 Aug;39(8):1647–1654. doi: 10.1128/aac.39.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Parikh K. In-vivo activity of streptomycin and clofazimine against established infections of Mycobacterium avium complex in beige mice. J Antimicrob Chemother. 1992 Dec;30(6):833–838. doi: 10.1093/jac/30.6.833. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Farhi D. C., LaBrecque J. The beige mouse model for Mycobacterium avium complex (MAC) disease: optimal conditions for the host and parasite. Tubercle. 1989 Dec;70(4):257–271. doi: 10.1016/0041-3879(89)90020-2. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Parikh K., Podapati N. R., Taylor R., Farhi D. C., Iseman M. D. Susceptibility of beige mice to Mycobacterium avium complex infections by different routes of challenge. Am Rev Respir Dis. 1989 May;139(5):1098–1104. doi: 10.1164/ajrccm/139.5.1098. [DOI] [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Ji B., Lounis N., Truffot-Pernot C., Grosset J. Effectiveness of various antimicrobial agents against Mycobacterium avium complex in the beige mouse model. Antimicrob Agents Chemother. 1994 Nov;38(11):2521–2529. doi: 10.1128/aac.38.11.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens S. P., DeStefano M. S., Cynamon M. H. Activity of clarithromycin against Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1992 Nov;36(11):2413–2417. doi: 10.1128/aac.36.11.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney K. H., Morse S. S., Morahan P. S. Macrophage functions in beige (Chédiak-Higashi syndrome) mice. Cancer Res. 1980 Nov;40(11):3934–3939. [PubMed] [Google Scholar]
- Orme I. M., Furney S. K., Roberts A. D. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retroviruses. Infect Immun. 1992 Nov;60(11):4747–4753. doi: 10.1128/iai.60.11.4747-4753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa J., Flórido M., Kunze Z. M., Castro A. G., Portaels F., McFadden J., Silva M. T., Appelberg R. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin Exp Immunol. 1994 Nov;98(2):210–216. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades E. R., Cooper A. M., Orme I. M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995 Oct;63(10):3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Sarmento A. M., Appelberg R. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect Immun. 1995 Oct;63(10):3759–3764. doi: 10.1128/iai.63.10.3759-3764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B. M., Cheers C. Inflammatory response following intranasal infection with Mycobacterium avium complex: role of T-cell subsets and gamma interferon. Infect Immun. 1995 Jun;63(6):2282–2287. doi: 10.1128/iai.63.6.2282-2287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]


