Abstract
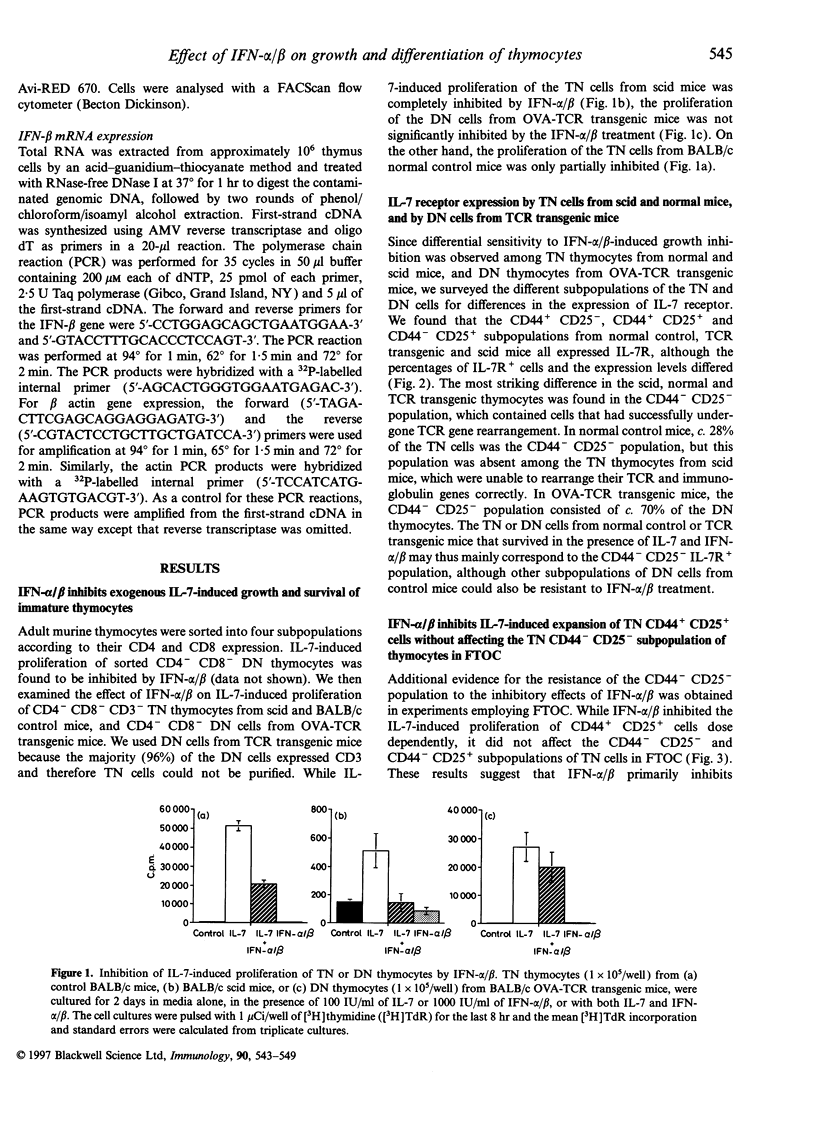
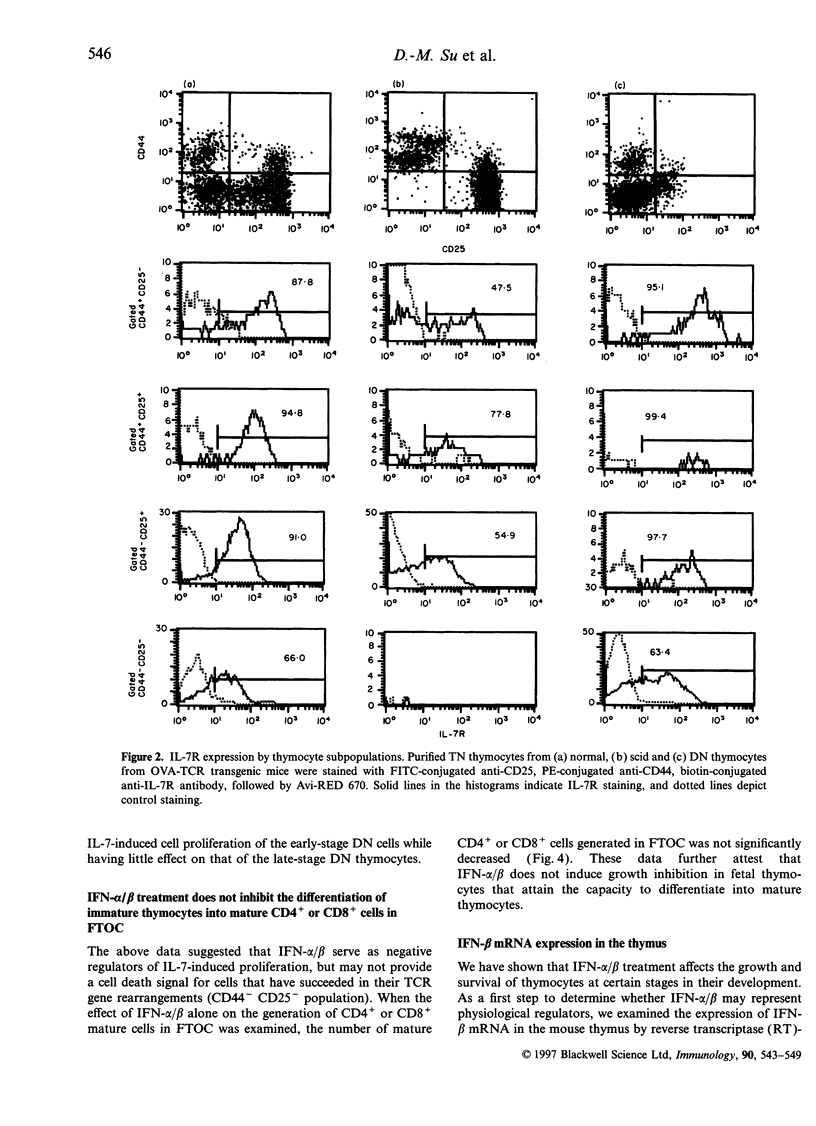
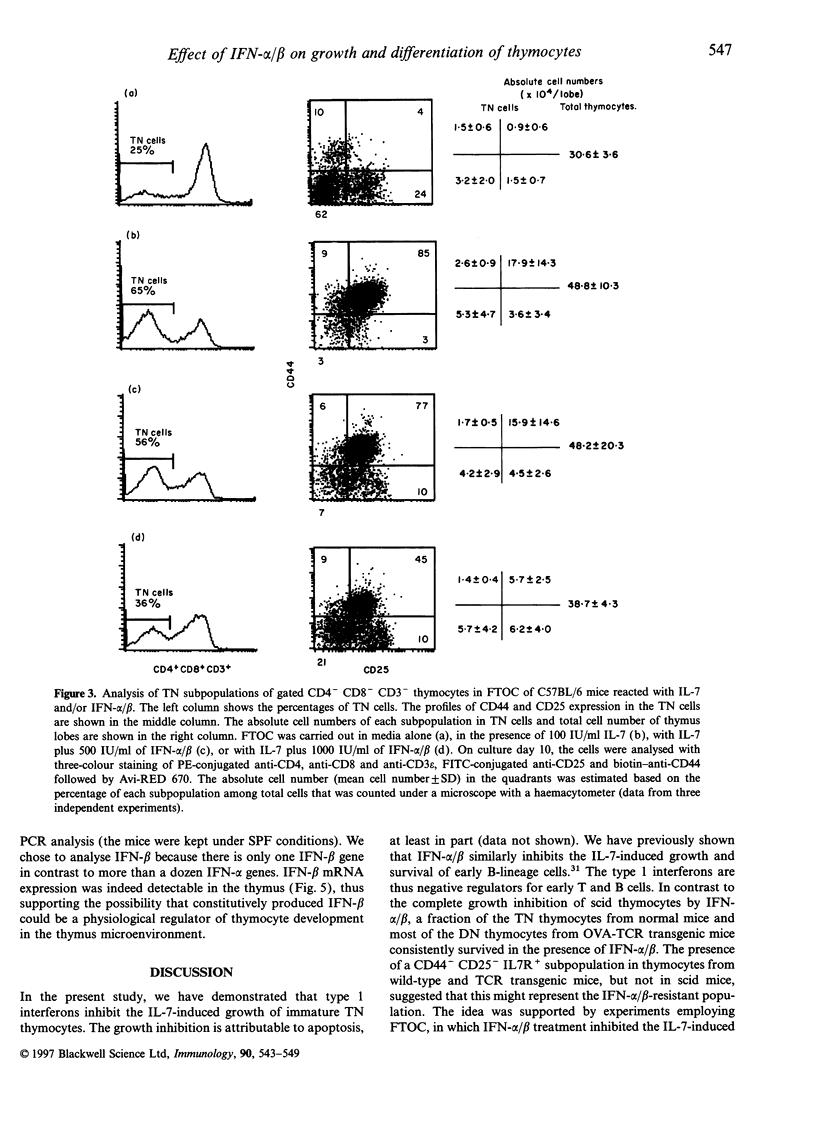
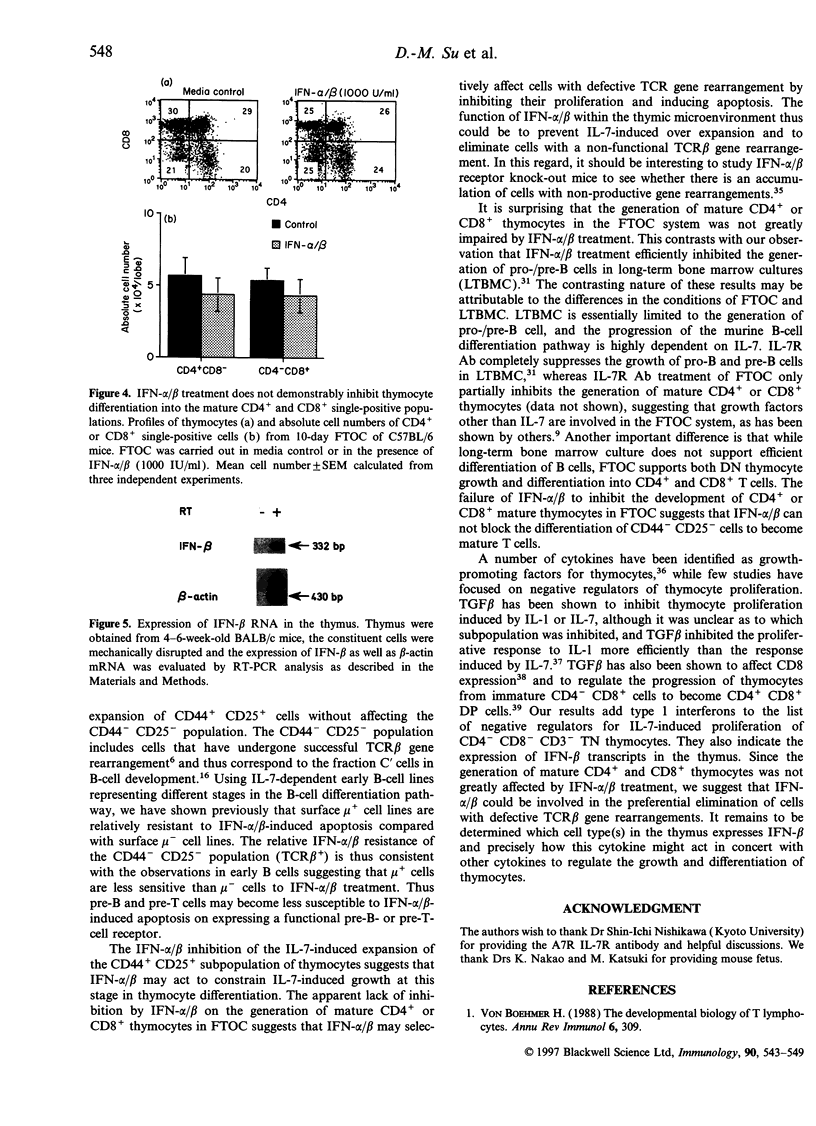
Type 1 interferons (IFN-alpha/beta) have recently been shown to inhibit interleukin-7 (IL-7)-induced growth and survival of early B-lineage cells. The CD3- CD4- CD8- (triple negative; TN) thymocytes from normal mice strongly proliferated upon stimulation with IL-7 in suspension, culture. Such an IL-7-induced proliferation was suppressed by the addition of IFN-alpha/beta, but a fraction of the TN thymocytes still showed proliferation. The IL-7-induced growth of TN thymocytes from acid mice, which lack the CD44- CD25- subpopulation, was completely inhibited by the addition of IFN-alpha/beta. The IL-7 induced proliferation of CD4- CD8- thymocytes from T-cell receptor (TCR) transgenic mice, the majority of which are CD3+ CD44- CD25-, was resistant to IFN-alpha/beta-mediated suppression. In fetal thymus organ cultures (FTOC), the addition of IL-7 greatly increased the population of CD4- CD8- CD44+ CD25+ thymocytes and IFN-alpha/beta inhibited this IL-7-driven expansion. In contrast, the addition of IL-7 markedly decreased the percentages of CD4- CD8- CD3- CD44- CD25- cells, and IFN-alpha/beta reversed the effect and increased the subpopulations of CD44- CD25+ and CD44- CD25-. Finally, IFN-beta mRNA was found to be expressed in the thymus. The data suggest that type I interferons inhibit IL-7-driven proliferation of TN thymocytes, but do not block the normal differentiation process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Bödeker B. G., van Eijk R. V., Mühlradt P. F. Mitogenic effects of partially purified interleukin 2 on thymocyte subpopulations and spleen t cells of the mouse. Eur J Immunol. 1980 Sep;10(9):702–707. doi: 10.1002/eji.1830100909. [DOI] [PubMed] [Google Scholar]
- Carding S. R., Hayday A. C., Bottomly K. Cytokines in T-cell development. Immunol Today. 1991 Jul;12(7):239–245. doi: 10.1016/0167-5699(91)90037-T. [DOI] [PubMed] [Google Scholar]
- Chantry D., Turner M., Feldmann M. Interleukin 7 (murine pre-B cell growth factor/lymphopoietin 1) stimulates thymocyte growth: regulation by transforming growth factor beta. Eur J Immunol. 1989 Apr;19(4):783–786. doi: 10.1002/eji.1830190433. [DOI] [PubMed] [Google Scholar]
- Dudley E. C., Petrie H. T., Shah L. M., Owen M. J., Hayday A. C. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994 May;1(2):83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Ezine S., Ceredig R. Haemopoiesis and early T-cell differentiation. Immunol Today. 1994 Apr;15(4):151–154. doi: 10.1016/0167-5699(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I., Kennedy J., Mombaerts P., Tonegawa S., Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8- thymocyte differentiation. J Immunol. 1994 May 15;152(10):4783–4792. [PubMed] [Google Scholar]
- Godfrey D. I., Kennedy J., Suda T., Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993 May 15;150(10):4244–4252. [PubMed] [Google Scholar]
- Godfrey D. I., Zlotnik A. Control points in early T-cell development. Immunol Today. 1993 Nov;14(11):547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Waldschmidt T. J., Finkelman F. D., Hess B. W., Alpert A. R., Boiani N. E., Namen A. E., Morrissey P. J. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993 Jul 1;178(1):257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groettrup M., Ungewiss K., Azogui O., Palacios R., Owen M. J., Hayday A. C., von Boehmer H. A novel disulfide-linked heterodimer on pre-T cells consists of the T cell receptor beta chain and a 33 kd glycoprotein. Cell. 1993 Oct 22;75(2):283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- Groettrup M., von Boehmer H. A role for a pre-T-cell receptor in T-cell development. Immunol Today. 1993 Dec;14(12):610–614. doi: 10.1016/0167-5699(93)90201-U. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbelin A., Machavoine F., Vicari A., Schneider E., Papiernik M., Ziltener H., Penit C., Dy M. Endogenous granulocyte-macrophage colony-stimulating factor is involved in IL-1- and IL-7-induced murine thymocyte proliferation. J Immunol. 1994 Sep 1;153(5):1973–1981. [PubMed] [Google Scholar]
- Iwabuchi K., Nakayama K., McCoy R. L., Wang F., Nishimura T., Habu S., Murphy K. M., Loh D. Y. Cellular and peptide requirements for in vitro clonal deletion of immature thymocytes. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9000–9004. doi: 10.1073/pnas.89.19.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi H., Su D. M., Muraguchi A., Watanabe T. A novel cell surface antigen, immature thymocyte antigen-1, is involved in the differentiation of murine thymocytes. J Immunol. 1995 Jul 15;155(2):568–577. [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Moore T. A., Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995 Sep 1;86(5):1850–1860. [PubMed] [Google Scholar]
- Murray R., Suda T., Wrighton N., Lee F., Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;1(5):526–531. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- Müller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994 Jun 24;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Peschon J. J., Morrissey P. J., Grabstein K. H., Ramsdell F. J., Maraskovsky E., Gliniak B. C., Park L. S., Ziegler S. F., Williams D. E., Ware C. B. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994 Nov 1;180(5):1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum J., De Smedt M., Leclercq G. Exogenous IL-7 promotes the growth of CD3-CD4-CD8-CD44+CD25+/- precursor cells and blocks the differentiation pathway of TCR-alpha beta cells in fetal thymus organ culture. J Immunol. 1993 Apr 1;150(7):2706–2716. [PubMed] [Google Scholar]
- Ranges G. E., Zlotnik A., Espevik T., Dinarello C. A., Cerami A., Palladino M. A., Jr Tumor necrosis factor alpha/cachectin is a growth factor for thymocytes. Synergistic interactions with other cytokines. J Exp Med. 1988 Apr 1;167(4):1472–1478. doi: 10.1084/jem.167.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E. V., Chen D., Diamond R. A. Functional and phenotypic analysis of thymocytes in SCID mice. Evidence for functional response transitions before and after the SCID arrest point. J Immunol. 1993 Oct 1;151(7):3530–3546. [PubMed] [Google Scholar]
- Saint-Ruf C., Ungewiss K., Groettrup M., Bruno L., Fehling H. J., von Boehmer H. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994 Nov 18;266(5188):1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- Suda T., Murray R., Guidos C., Zlotnik A. Growth-promoting activity of IL-1 alpha, IL-6, and tumor necrosis factor-alpha in combination with IL-2, IL-4, or IL-7 on murine thymocytes. Differential effects on CD4/CD8 subsets and on CD3+/CD3- double-negative thymocytes. J Immunol. 1990 Apr 15;144(8):3039–3045. [PubMed] [Google Scholar]
- Suda T., Zlotnik A. IL-7 maintains the T cell precursor potential of CD3-CD4-CD8- thymocytes. J Immunol. 1991 May 1;146(9):3068–3073. [PubMed] [Google Scholar]
- Suda T., Zlotnik A. In vitro induction of CD8 expression on thymic pre-T cells. I. Transforming growth factor-beta and tumor necrosis factor-alpha induce CD8 expression on CD8- thymic subsets including the CD25+CD3-CD4-CD8- pre-T cell subset. J Immunol. 1992 Mar 15;148(6):1737–1745. [PubMed] [Google Scholar]
- Sudo T., Nishikawa S., Ohno N., Akiyama N., Tamakoshi M., Yoshida H., Nishikawa S. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama Y., Letterio J. J., Suzuki H., Farr A. G., Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J Exp Med. 1994 May 1;179(5):1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari A. P., Bean A. G., Zlotnik A. A role for BP-3/BST-1 antigen in early T cell development. Int Immunol. 1996 Feb;8(2):183–191. doi: 10.1093/intimm/8.2.183. [DOI] [PubMed] [Google Scholar]
- Vissinga C. S., Fatur-Saunders D. J., Takei F. Dual role of IL-7 in the growth and differentiation of immature thymocytes. Exp Hematol. 1992 Sep;20(8):998–1003. [PubMed] [Google Scholar]
- Wang J., Lin Q., Langston H., Cooper M. D. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995 Oct;3(4):475–484. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Watson J. D., Morrissey P. J., Namen A. E., Conlon P. J., Widmer M. B. Effect of IL-7 on the growth of fetal thymocytes in culture. J Immunol. 1989 Aug 15;143(4):1215–1222. [PubMed] [Google Scholar]
- Zlotnik A., Ransom J., Frank G., Fischer M., Howard M. Interleukin 4 is a growth factor for activated thymocytes: possible role in T-cell ontogeny. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3856–3860. doi: 10.1073/pnas.84.11.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]



