Abstract
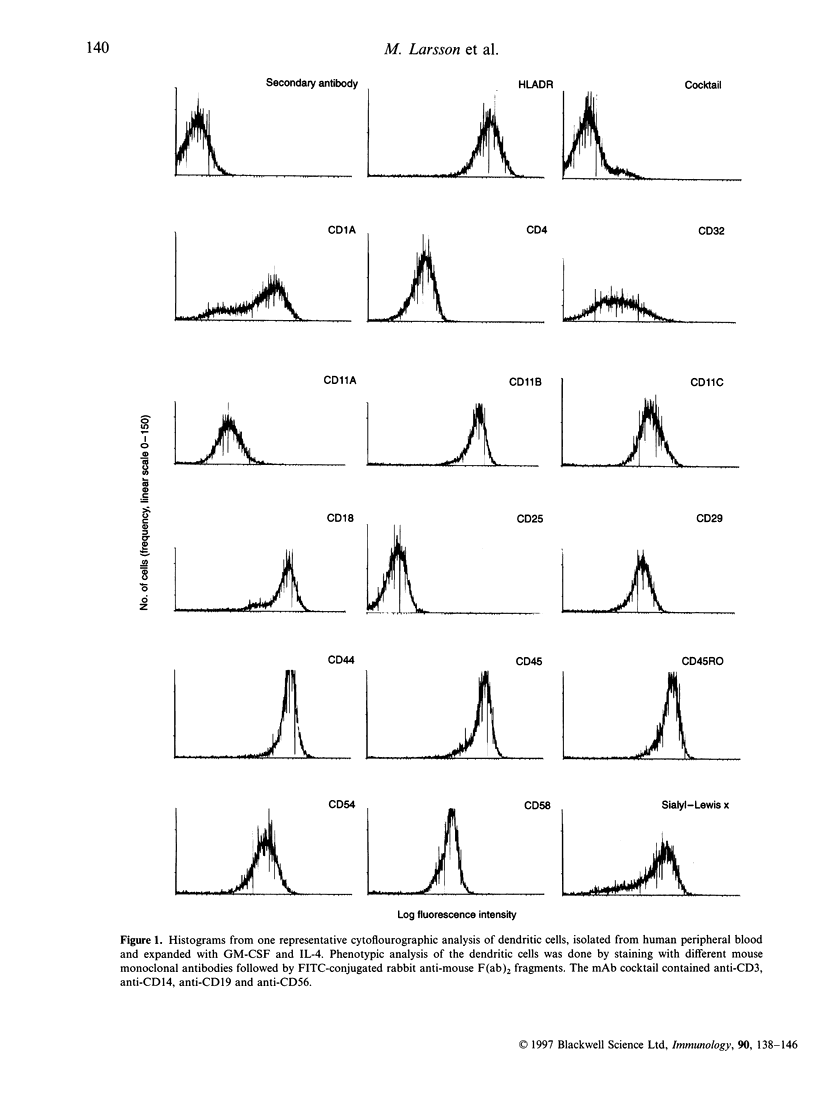
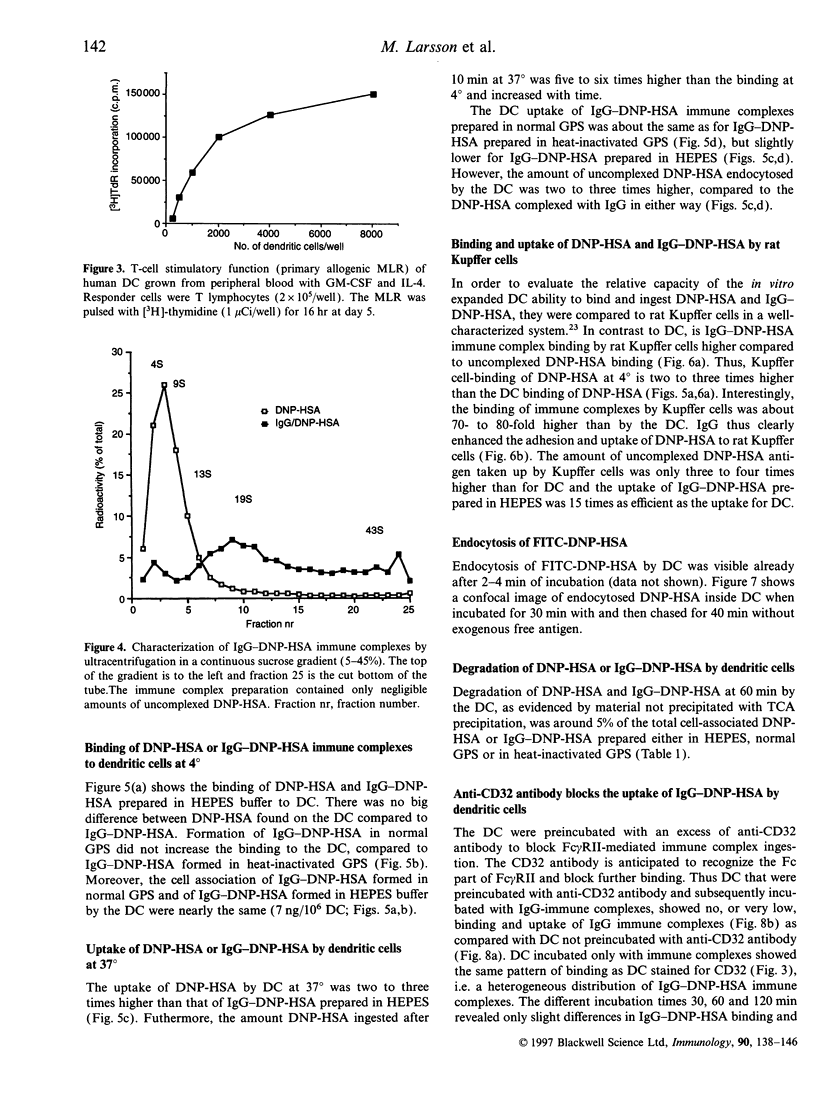
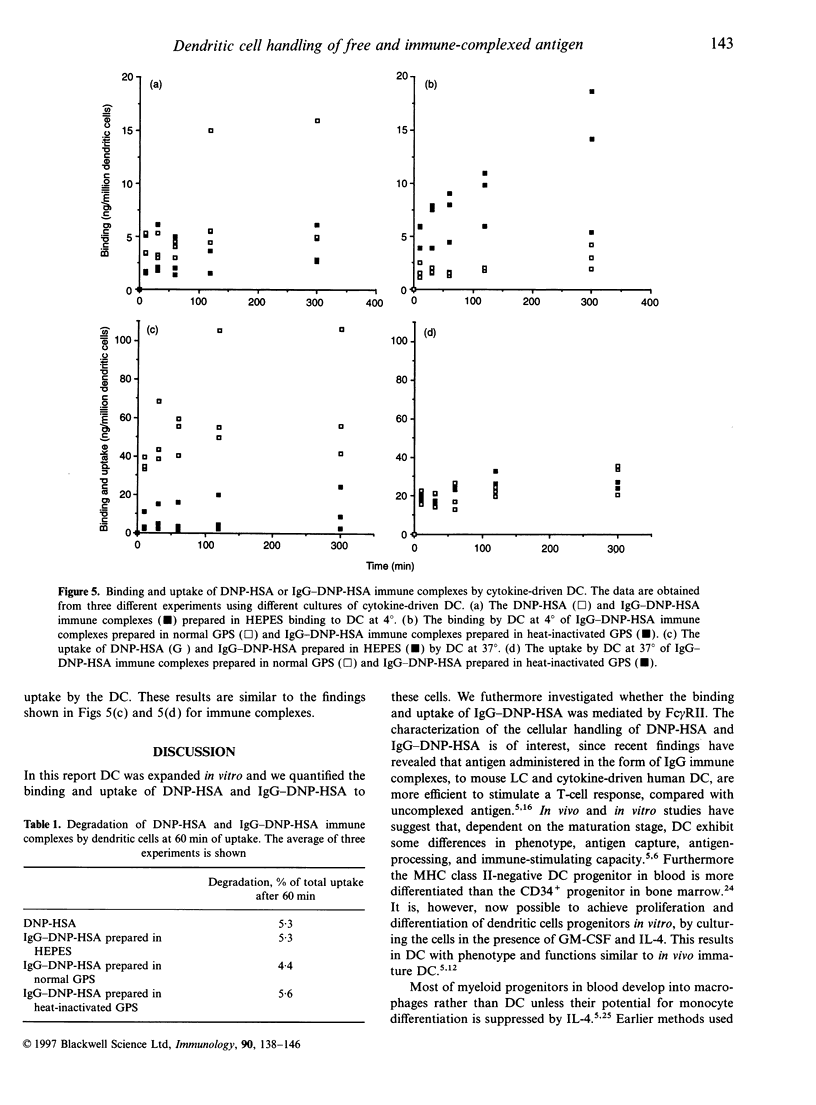
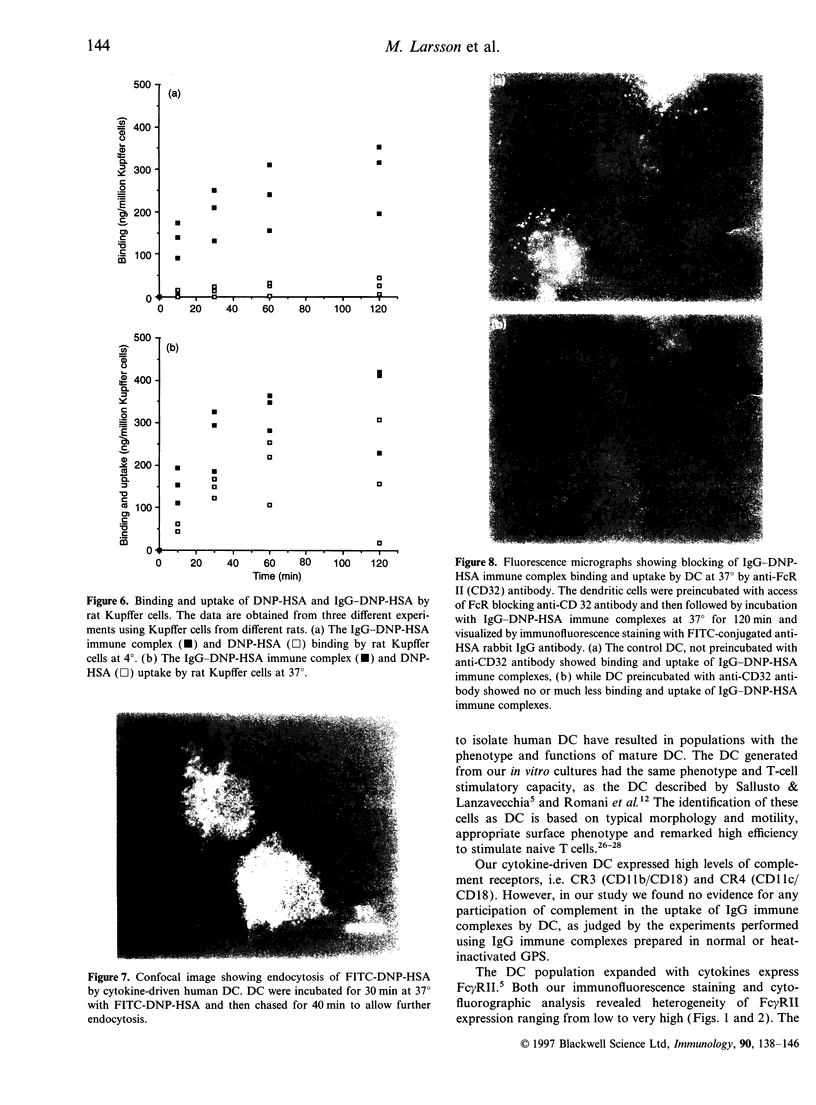
The handling of free and IgG-complexed dinitrophenylated human serum albumin (DNP-HSA) by human dendritic cells (DC) cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) was studied. It has been shown that the amount of uncomplexed or IgG-complexed antigen required by DC to start an immune response is low compared with other antigen-presenting cells. We therefore examined whether such efficient presentation of immune complexes is due to an enhanced Fc gamma RII-mediated endocytosis or to a specialized and efficient antigen handling, i.e., macropinocytosis. The Fc gamma RII expression was found to be heterogeneous on the GM-CSF- and IL-4-cultured DC, i.e. it ranges from low to high expression. The handling of antigen and immune complexes revealed, that the level of binding and uptake of IgG-DNP-HSA complexes by in vitro expanded DC is low compared with free antigen. Uncomplexed DNP-HSA is probably handled either by endocytosis via receptors being more abundant and/or efficient than the Fc gamma RII or via non-receptor-mediated endocytosis. The binding and uptake of IgG-complexed DNP-HSA was blocked by anti-Fc gamma RII antibody, indicating the specificity of the interaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks D. G., Qiu W. Q., Luster A. D., Ravetch J. V. Structure and expression of human IgG FcRII(CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J Exp Med. 1989 Oct 1;170(4):1369–1385. doi: 10.1084/jem.170.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M., Duncan D. D., Swain S. L. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992 Nov 1;176(5):1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito-Farese M. E., Sautès C., de la Salle H., Latour S., Bieber T., de la Salle C., Ohlmann P., Fridman W. H., Cazenave J. P., Teillaud J. L. Membrane and soluble Fc gamma RII/III modulate the antigen-presenting capacity of murine dendritic epidermal Langerhans cells for IgG-complexed antigens. J Immunol. 1995 Aug 15;155(4):1725–1736. [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Romani N., Steinman R. M. An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J Exp Med. 1989 Aug 1;170(2):527–542. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik Z. K., Park J. G., Hunter S., Schreiber A. D. Structure/function relationships of Fc gamma receptors in phagocytosis. Semin Immunol. 1995 Feb;7(1):45–54. doi: 10.1016/1044-5323(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Jansen J. H., Wientjens G. J., Fibbe W. E., Willemze R., Kluin-Nelemans H. C. Inhibition of human macrophage colony formation by interleukin 4. J Exp Med. 1989 Aug 1;170(2):577–582. doi: 10.1084/jem.170.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A. G., Skogh T. Different elimination of circulating IgA immune complexes in rat and guinea pig. Blood clearance, organ distribution and cellular uptake in the liver. Int Arch Allergy Immunol. 1994;103(1):36–43. doi: 10.1159/000236603. [DOI] [PubMed] [Google Scholar]
- Ljunghusen O., Johansson A., Skogh T. Hepatic immune complex elimination studied with FITC-labelled antigen. J Immunol Methods. 1990 Mar 27;128(1):1–7. doi: 10.1016/0022-1759(90)90457-7. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., De Bruijn M. L., Boog C. J., Nieland J. D., Boes J., Melief C. J., Ploegh H. L. N-linked glycan modification on antigen-presenting cells restores an allospecific cytotoxic T cell response. J Exp Med. 1990 Feb 1;171(2):583–588. doi: 10.1084/jem.171.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman H. W., Kleijmeer M. J., Ossevoort M. A., Oorschot V. M., Vierboom M. P., van de Keur M., Kenemans P., Kast W. M., Geuze H. J., Melief C. J. Antigen capture and major histocompatibility class II compartments of freshly isolated and cultured human blood dendritic cells. J Exp Med. 1995 Jul 1;182(1):163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Peng M., Gezelter S., Swiggard W. J., Betjes M., Bhardwaj N., Steinman R. M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994 Jul;82(3):487–493. [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M., Betjes M. G., Hirmand H., Hoffman L., Steinman R. M. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J Invest Dermatol. 1995 Jan;104(1):11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- Reid C. D., Fryer P. R., Clifford C., Kirk A., Tikerpae J., Knight S. C. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood. 1990 Sep 15;76(6):1139–1149. [PubMed] [Google Scholar]
- Reid C. D., Stackpoole A., Meager A., Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992 Oct 15;149(8):2681–2688. [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kämpgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P. O., Steinman R. M., Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994 Jul 1;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995 Aug 1;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994 Apr 1;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Rappa D. A., Laky K., Carsons S. E. Stem cell factor augments tumor necrosis factor-granulocyte-macrophage colony-stimulating factor-mediated dendritic cell hematopoiesis. Stem Cells. 1995 Mar;13(2):186–197. doi: 10.1002/stem.5530130210. [DOI] [PubMed] [Google Scholar]
- Skogh T., Magnusson K. E., Stendahl O. Hydrophobic interaction between DNP-HSA and different tissue structures in vivo assessed by indirect immunofluorescence microscopy. J Immunol Methods. 1983 Jul 29;61(3):385–390. doi: 10.1016/0022-1759(83)90235-1. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Antigen-presenting cells for CD8+ T cells. Immunol Rev. 1990 Oct;117:213–234. doi: 10.1111/j.1600-065x.1990.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Stahl P. D. The mannose receptor and other macrophage lectins. Curr Opin Immunol. 1992 Feb;4(1):49–52. doi: 10.1016/0952-7915(92)90123-v. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Szabolcs P., Moore M. A., Young J. W. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995 Jun 1;154(11):5851–5861. [PubMed] [Google Scholar]
- Tax W. J., van de Winkel J. G. Human Fc gamma receptor II: a standby receptor activated by proteolysis? Immunol Today. 1990 Sep;11(9):308–310. doi: 10.1016/0167-5699(90)90125-s. [DOI] [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Williams L. A., Egner W., Hart D. N. Isolation and function of human dendritic cells. Int Rev Cytol. 1994;153:41–103. doi: 10.1016/s0074-7696(08)62188-9. [DOI] [PubMed] [Google Scholar]