Abstract
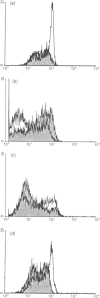
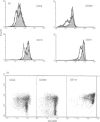
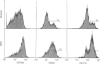
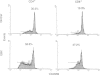
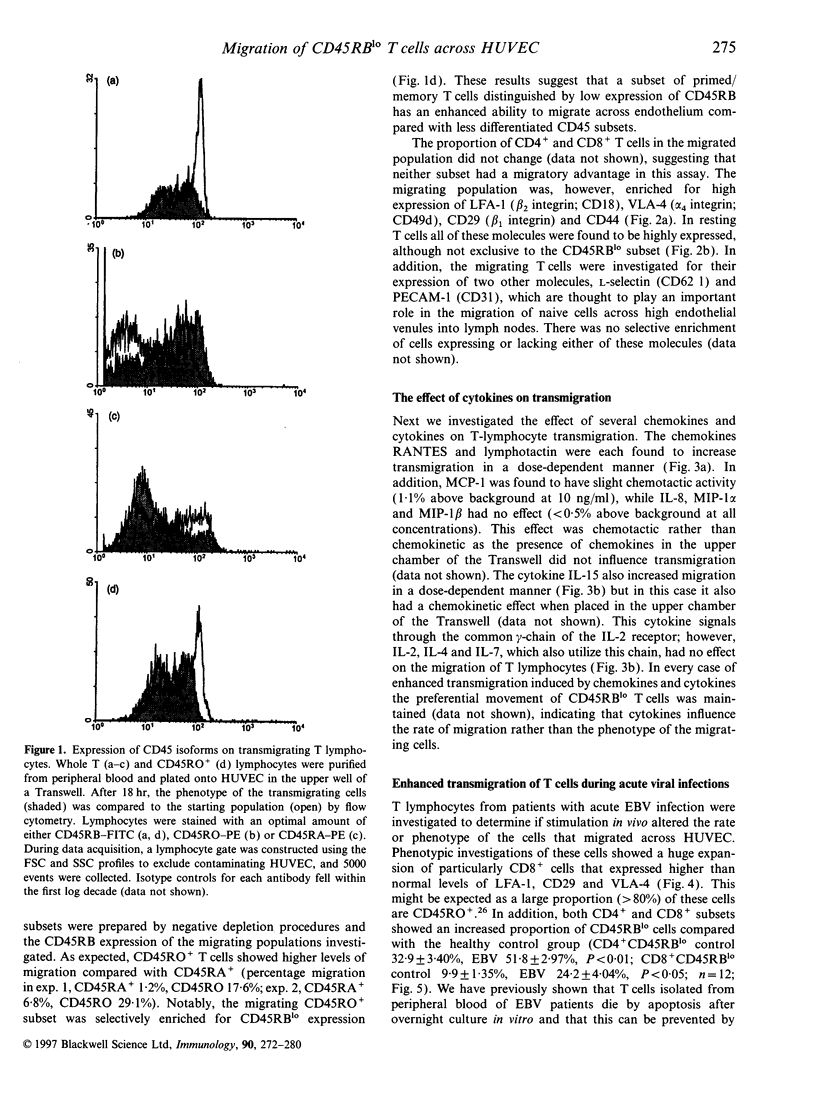
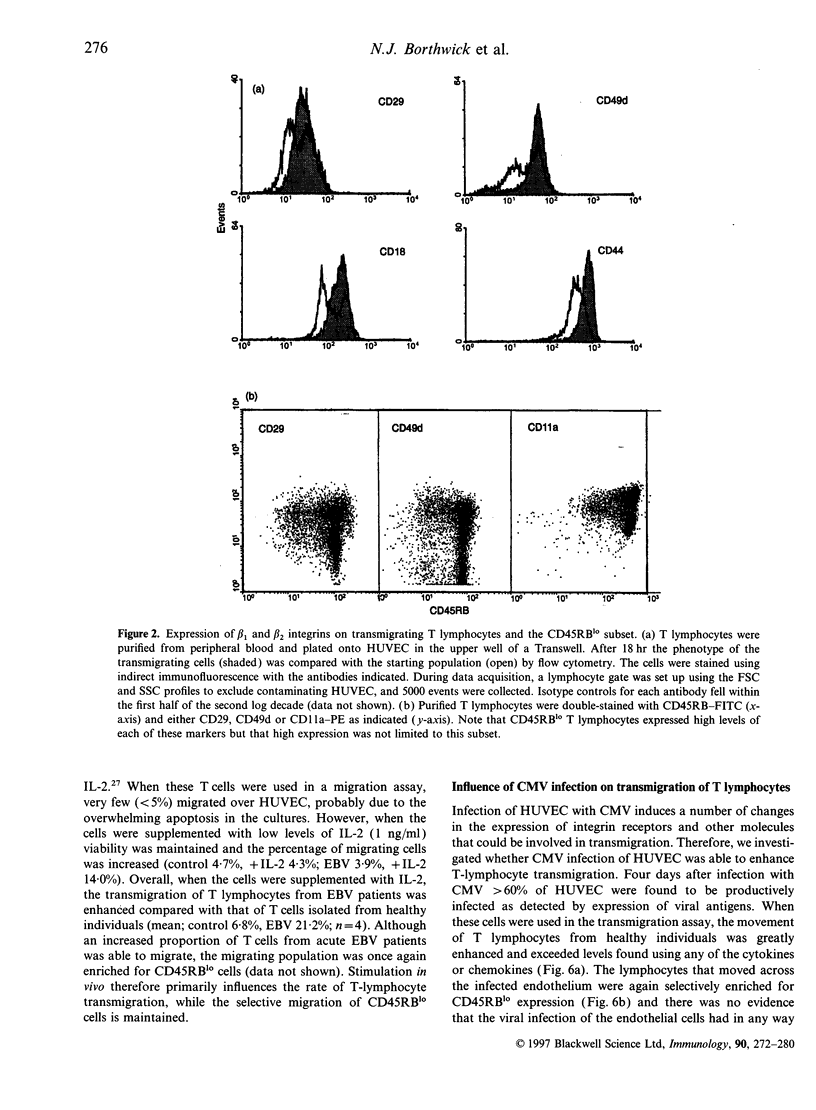
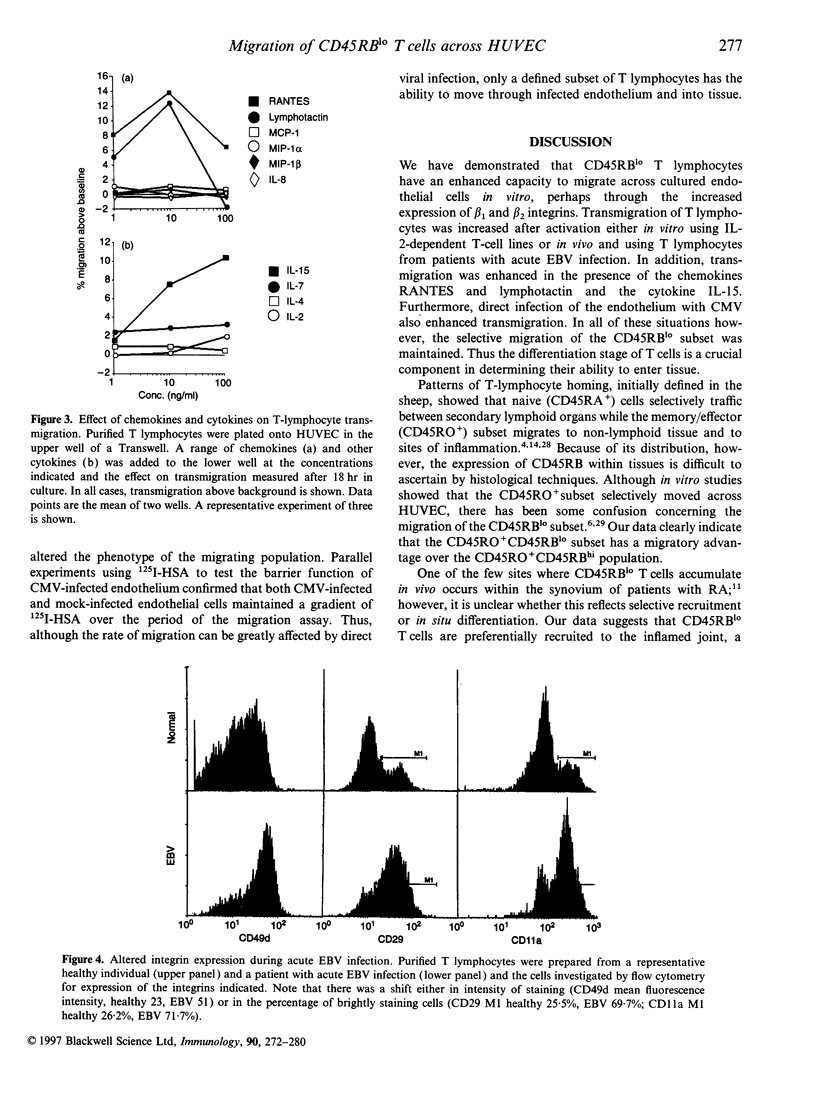
Low expression of CD45RB on CD45RO+ T lymphocytes defines a subset of highly differentiated T lymphocytes that accumulate in vivo within the affected joints of patients with rheumatoid arthritis (RA). Although it is known that CD45RO+ T lymphocytes migrate to sites of inflammation in vivo, it is not clear whether within this subset the CD45RBlo cells are selectively recruited or develop in situ within the joint. Using a transwell system we show that a small proportion of resting T lymphocytes migrated across unactivated human umbilical vein endothelial cells (HUVEC). These migrating cells were CD45RO+ and enriched for low CD45RB expression. In addition, both the CD45RO+CD45RBlo subset and migrating cells expressed increased levels of beta 1 and beta 2 integrins and CD44. The percentage of CD45RO+CD45RBlo T lymphocytes was increased in the circulation of patients with acute Epstein-Barr virus (EBV) infection. These in vivo activated cells also expressed increased levels beta 1 and beta 2 integrins and CD44, and showed an enhanced rate of transmigration compared with resting T lymphocytes. Transmigration of T lymphocytes was increased using the chemokines RANTES and lymphotactin and the cytokine interleukin-15 (IL-15). In addition, infection of the HUVEC with cytomegalovirus (CMV) led to an enhanced movement of T lymphocytes. In all of these cases the selective migration of the CD45RBlo subset was maintained. Thus although the rate of T-lymphocyte transmigration could be influenced by a number factors, the CD45RO+CD45RBlo subset has a migratory advantage suggesting that more differentiated CD45RO+CD45RBlo T lymphocytes are selectively recruited to sites of inflammation.
Full text
PDF


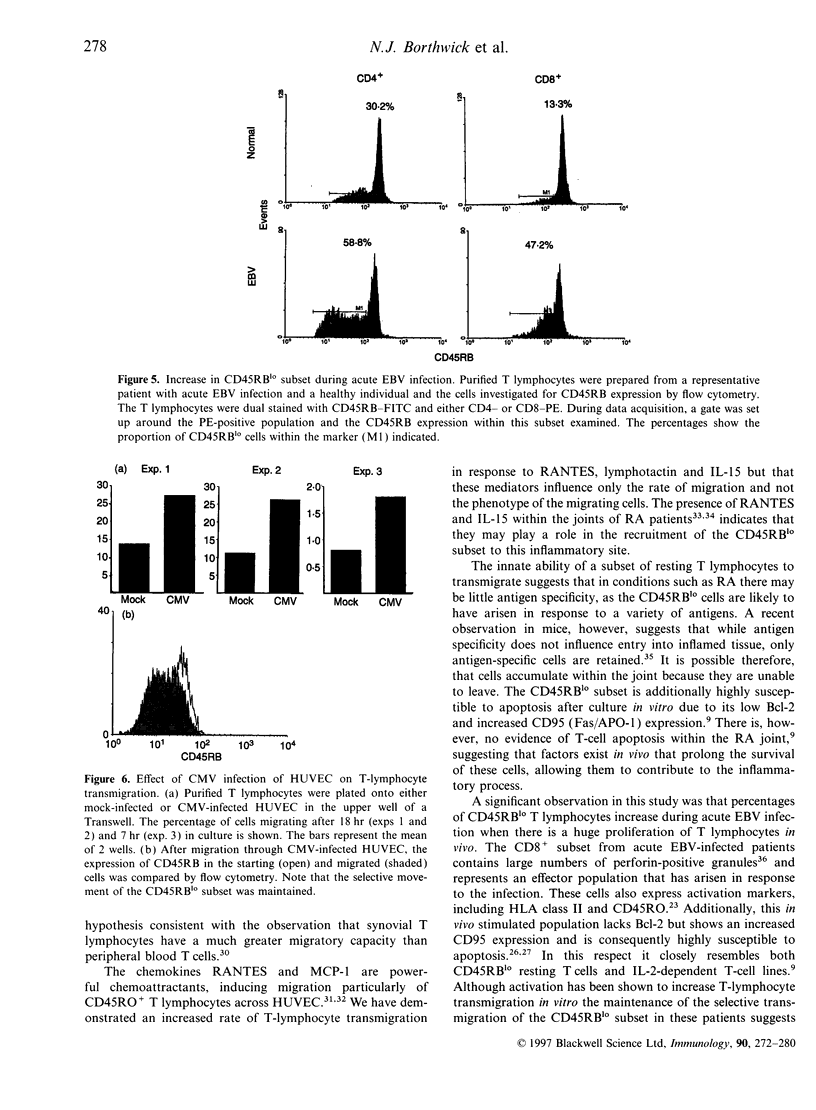


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Amlot P. L., Timms A., Lombardi G., Lechler R., Janossy G. The development of primed/memory CD8+ lymphocytes in vitro and in rejecting kidneys after transplantation. Clin Exp Immunol. 1990 Aug;81(2):225–231. doi: 10.1111/j.1365-2249.1990.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A. N., Borthwick N., Salmon M., Gombert W., Bofill M., Shamsadeen N., Pilling D., Pett S., Grundy J. E., Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A. N., Savill J., Gombert W., Bofill M., Borthwick N. J., Whitelaw F., Grundy J., Janossy G., Salmon M. The specific recognition by macrophages of CD8+,CD45RO+ T cells undergoing apoptosis: a mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994 Nov 1;180(5):1943–1947. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Bird I. N., Spragg J. H., Ager A., Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993 Dec;80(4):553–560. [PMC free article] [PubMed] [Google Scholar]
- Borthwick N. J., Bofill M., Hassan I., Panayiotidis P., Janossy G., Salmon M., Akbar A. N. Factors that influence activated CD8+ T-cell apoptosis in patients with acute herpesvirus infections: loss of costimulatory molecules CD28, CD5 and CD6 but relative maintenance of Bax and Bcl-X expression. Immunology. 1996 Aug;88(4):508–515. [PMC free article] [PubMed] [Google Scholar]
- Brezinschek R. I., Lipsky P. E., Galea P., Vita R., Oppenheimer-Marks N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J Immunol. 1995 Apr 1;154(7):3062–3077. [PubMed] [Google Scholar]
- Carr M. W., Roth S. J., Luther E., Rose S. S., Springer T. A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Pietschmann P., Oppenheimer-Marks N., Lipsky P. E. The intrinsic migratory capacity of memory T cells contributes to their accumulation in rheumatoid synovium. Arthritis Rheum. 1992 Dec;35(12):1434–1444. doi: 10.1002/art.1780351206. [DOI] [PubMed] [Google Scholar]
- Galéa P., Brezinschek R., Lipsky P. E., Oppenheimer-Marks N. Phenotypic characterization of CD4-/alpha beta TCR+ and gamma delta TCR+ T cells with a transendothelial migratory capacity. J Immunol. 1994 Jul 15;153(2):529–542. [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med. 1995;6(2):109–118. doi: 10.1177/10454411950060020101. [DOI] [PubMed] [Google Scholar]
- Horgan K. J., Tanaka Y., Luce G. E., van Seventer G. A., Nutman T. B., Shaw S. CD45RB expression defines two interconvertible subsets of human CD4+ T cells with memory function. Eur J Immunol. 1994 May;24(5):1240–1243. doi: 10.1002/eji.1830240536. [DOI] [PubMed] [Google Scholar]
- Imhof B. A., Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- Irani D. N., Griffin D. E. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J Immunol. 1996 May 15;156(10):3850–3857. [PubMed] [Google Scholar]
- Jones D. A., McIntire L. V., Smith C. W., Picker L. J. A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J Clin Invest. 1994 Dec;94(6):2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohem C. L., Brezinschek R. I., Wisbey H., Tortorella C., Lipsky P. E., Oppenheimer-Marks N. Enrichment of differentiated CD45RBdim,CD27- memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996 May;39(5):844–854. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- Laffón A., García-Vicuña R., Humbría A., Postigo A. A., Corbí A. L., de Landázuri M. O., Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991 Aug;88(2):546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Marston W., Dudler L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol. 1992 Sep;22(9):2205–2210. doi: 10.1002/eji.1830220904. [DOI] [PubMed] [Google Scholar]
- Mason D., Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology. 1990 Aug;70(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- Masuyama J., Berman J. S., Cruikshank W. W., Morimoto C., Center D. M. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992 Mar 1;148(5):1367–1374. [PubMed] [Google Scholar]
- Matthews N., Emery P., Pilling D., Akbar A., Salmon M. Subpopulations of primed T helper cells in rheumatoid arthritis. Arthritis Rheum. 1993 May;36(5):603–607. doi: 10.1002/art.1780360505. [DOI] [PubMed] [Google Scholar]
- McInnes I. B., al-Mughales J., Field M., Leung B. P., Huang F. P., Dixon R., Sturrock R. D., Wilkinson P. C., Liew F. Y. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996 Feb;2(2):175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Adam E., DeBakey M. E. Possible role of cytomegalovirus in atherogenesis. JAMA. 1990 Apr 25;263(16):2204–2207. [PubMed] [Google Scholar]
- Miyawaki T., Kasahara Y., Kanegane H., Ohta K., Yokoi T., Yachie A., Taniguchi N. Expression of CD45R0 (UCHL1) by CD4+ and CD8+ T cells as a sign of in vivo activation in infectious mononucleosis. Clin Exp Immunol. 1991 Mar;83(3):447–451. doi: 10.1111/j.1365-2249.1991.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankonin G., Reipert B., Ager A. Interactions between interleukin-2-activated lymphocytes and vascular endothelium: binding to and migration across specialized and non-specialized endothelia. Immunology. 1992 Sep;77(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J. Control of lymphocyte homing. Curr Opin Immunol. 1994 Jun;6(3):394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Pietschmann P., Cush J. J., Lipsky P. E., Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992 Aug 15;149(4):1170–1178. [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G. H., Covelli M., Meliconi R., Markey A., Panayi G. S. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991 Feb;21(2):369–376. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Robinson E., Keystone E. C., Schall T. J., Gillett N., Fish E. N. Chemokine expression in rheumatoid arthritis (RA): evidence of RANTES and macrophage inflammatory protein (MIP)-1 beta production by synovial T cells. Clin Exp Immunol. 1995 Sep;101(3):398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. J., Carr M. W., Springer T. A. C-C chemokines, but not the C-X-C chemokines interleukin-8 and interferon-gamma inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur J Immunol. 1995 Dec;25(12):3482–3488. doi: 10.1002/eji.1830251241. [DOI] [PubMed] [Google Scholar]
- Salmon M., Pilling D., Borthwick N. J., Viner N., Janossy G., Bacon P. A., Akbar A. N. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994 Apr;24(4):892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- Scholz M., Hamann A., Blaheta R. A., Auth M. K., Encke A., Markus B. H. Cytomegalovirus- and interferon-related effects on human endothelial cells. Cytomegalovirus infection reduces upregulation of HLA class II antigen expression after treatment with interferon-gamma. Hum Immunol. 1992 Dec;35(4):230–238. doi: 10.1016/0198-8859(92)90004-7. [DOI] [PubMed] [Google Scholar]
- Wysocki J., Issekutz T. B. Effect of T cell activation on lymphocyte-endothelial cell adherence and the role of VLA-4 in the rat. Cell Immunol. 1992 Apr;140(2):420–431. doi: 10.1016/0008-8749(92)90208-7. [DOI] [PubMed] [Google Scholar]
- Yago T., Tsukuda M., Yamazaki H., Nishi T., Amano T., Minami M. Analysis of an initial step of T cell adhesion to endothelial monolayers under flow conditions. J Immunol. 1995 Feb 1;154(3):1216–1222. [PubMed] [Google Scholar]
- Yong K. L., Linch D. C. Granulocyte-macrophage-colony-stimulating factor differentially regulates neutrophil migration across IL-1-activated and nonactivated human endothelium. J Immunol. 1993 Mar 15;150(6):2449–2456. [PubMed] [Google Scholar]