Abstract
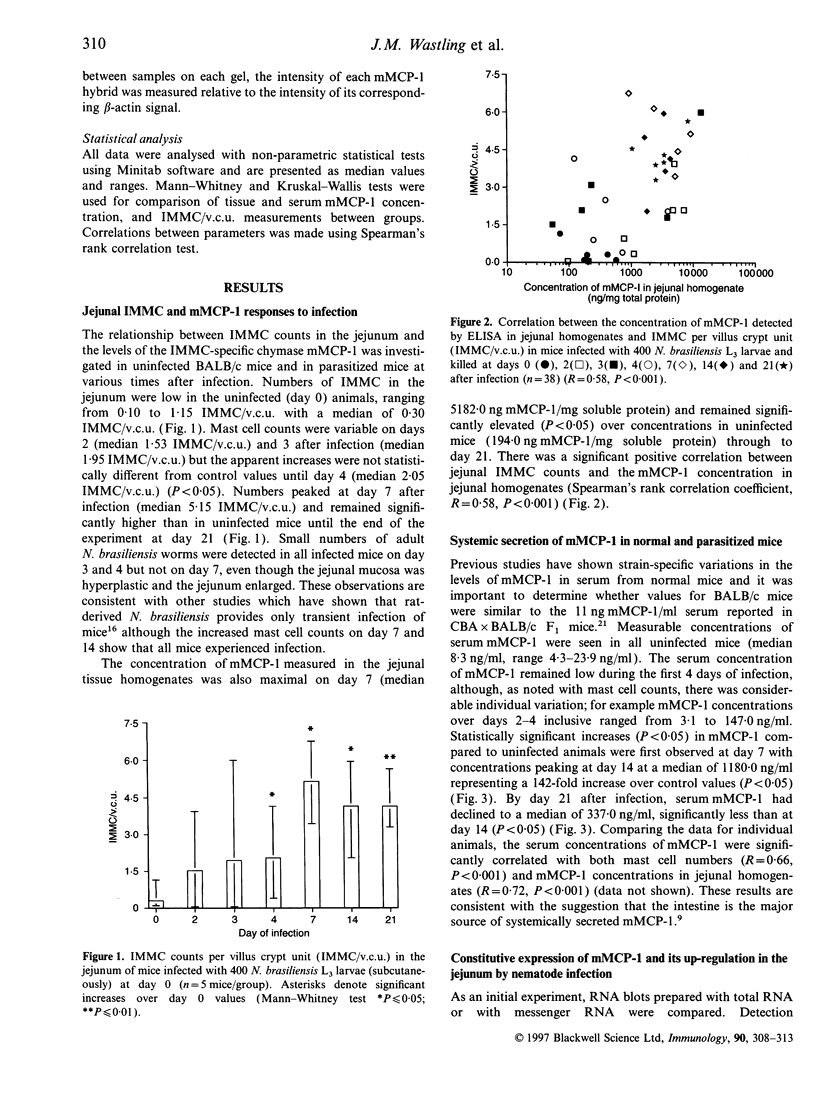
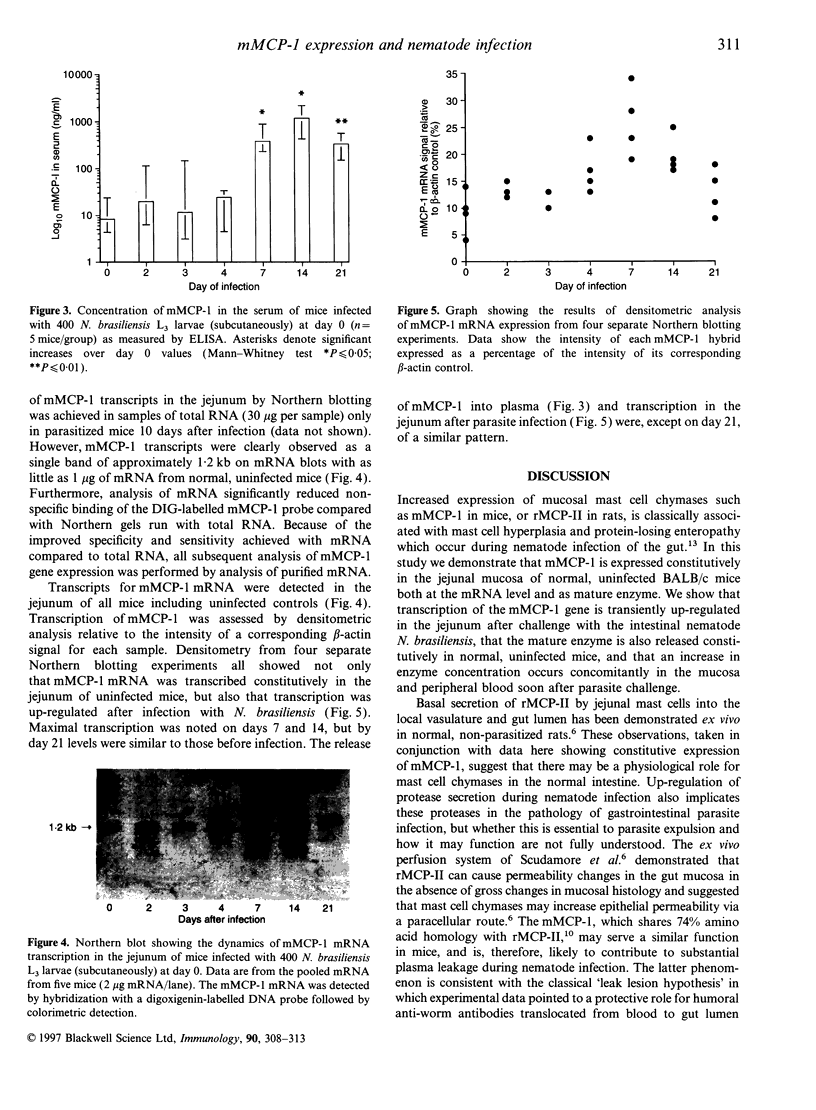
Rodent intestinal mucosal mast cells (IMMC) store and secrete soluble granule serine proteases, the beta-chymases, which may promote epithelial permeability during intestinal hypersensitivity reactions. The beta-chymase mouse mast cell protease-1 (mMCP-1) is generally considered to be expressed late in the in vitro differentiation of mast cells. The purpose of this study was to determine the kinetics of mMCP-1 transcription and expression in vivo during nematode-induced IMMC hyperplasia. Concentrations of mMCP-1 in blood and jejunum of BALB/c mice were quantified by enzyme-linked immunosorbent assay before and at various stages after infection with the intestinal nematode Nippostronglyus brasilliensis. Mature mMCP-1 enzyme was detected in jejunal homogenate (194 ng/mg soluble protein) and in blood (8.3 ng/ml serum) from normal uninfected BALB/c mice. Maximal IMMC hyperplasia occurred 7-14 days post infection and was significantly correlated with increased levels of mMCP-1 in jejunum (r = 0.58, P < 0.001) and with raised concentrations of mMCP-1 in serum (r = 0.66, P < 0.001). Transcription of the mMCP-1 gene was detected by RNA blotting in normal, uninfected jejunum, but transcription was up-regulated after infection with maximal transcription occurring on days 7 and 14. In conclusion, mMCP-1 transcription, storage and secretion occur constitutively in normal BALB/c jejunum but this basal secretion is up-regulated during nematode infection, suggesting both a physiological and pathological function for this protease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke J. M., Wahid F. N., Grencis R. K., Else K. J., Ben-Smith A. W., Goyal P. K. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): downregulation of specific cytokine secretion (IL-9 and IL-10) correlates with poor mastocytosis and chronic survival of adult worms. Parasite Immunol. 1993 Jul;15(7):415–421. doi: 10.1111/j.1365-3024.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan U. M., Sanker S., Glynias M. J., Karnik S. S., Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996 Jan 26;271(5248):502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- Cirino G., Cicala C., Bucci M. R., Sorrentino L., Maraganore J. M., Stone S. R. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996 Mar 1;183(3):821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson L. E., Schmitt E., Huntley J. F., Newlands G. F., Grencis R. K. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol. 1996 Apr;8(4):559–567. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Tsai M., Wershil B. K. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol. 1993 Apr;142(4):965–974. [PMC free article] [PubMed] [Google Scholar]
- Ghildyal N., McNeil H. P., Stechschulte S., Austen K. F., Silberstein D., Gurish M. F., Somerville L. L., Stevens R. L. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992 Sep 15;149(6):2123–2129. [PubMed] [Google Scholar]
- Grencis R. K. T cell and cytokine basis of host variability in response to intestinal nematode infections. Parasitology. 1996;112 (Suppl):S31–S37. [PubMed] [Google Scholar]
- Haig D. M., Huntley J. F., MacKellar A., Newlands G. F., Inglis L., Sangha R., Cohen D., Hapel A., Galli S. J., Miller H. R. Effects of stem cell factor (kit-ligand) and interleukin-3 on the growth and serine proteinase expression of rat bone-marrow-derived or serosal mast cells. Blood. 1994 Jan 1;83(1):72–83. [PubMed] [Google Scholar]
- Huang R. Y., Blom T., Hellman L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur J Immunol. 1991 Jul;21(7):1611–1621. doi: 10.1002/eji.1830210706. [DOI] [PubMed] [Google Scholar]
- Huntley J. F., Gooden C., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D., Tuohy M., Woodbury R. G., Miller H. R. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990 Jan;12(1):85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988 Dec;65(4):559–566. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Vet Immunol Immunopathol. 1984 May;6(1-2):167–259. doi: 10.1016/0165-2427(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Adoptive transfer of the intestinal mast cell response in rats infected with Nippostrongylus brasiliensis. Cell Immunol. 1979 Feb;42(2):225–239. doi: 10.1016/0008-8749(79)90188-6. [DOI] [PubMed] [Google Scholar]
- Newlands G. F., Gibson S., Knox D. P., Grencis R., Wakelin D., Miller H. R. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987 Dec;62(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., Lammas D. A., Huntley J. F., MacKellar A., Wakelin D., Miller H. R. Heterogeneity of murine bone marrow-derived mast cells: analysis of their proteinase content. Immunology. 1991 Mar;72(3):434–439. [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., Miller H. R., MacKellar A., Galli S. J. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995 Sep 1;86(5):1968–1976. [PubMed] [Google Scholar]
- Scudamore C. L., Thornton E. M., McMillan L., Newlands G. F., Miller H. R. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med. 1995 Dec 1;182(6):1871–1881. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Weis J. H. Mucosal T cells and mast cells share common adhesion receptors. Immunol Today. 1996 Feb;17(2):60–63. doi: 10.1016/0167-5699(96)80580-9. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Friend D. S., McNeil H. P., Schiller V., Ghildyal N., Austen K. F. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trong H. L., Newlands G. F., Miller H. R., Charbonneau H., Neurath H., Woodbury R. G. Amino acid sequence of a mouse mucosal mast cell protease. Biochemistry. 1989 Jan 10;28(1):391–395. doi: 10.1021/bi00427a054. [DOI] [PubMed] [Google Scholar]
- Warner J. A., Goldring K., Thomas L. H., Lavens S. E. Regulation of integrin-dependent release in human lung mast cells and basophils. Int Arch Allergy Immunol. 1995 May-Jun;107(1-3):151–153. doi: 10.1159/000236960. [DOI] [PubMed] [Google Scholar]
- Wershil B. K., Furuta G. T., Wang Z. S., Galli S. J. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996 May;110(5):1482–1490. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology. 1982 Jul;46(3):487–495. [PMC free article] [PubMed] [Google Scholar]



