Abstract
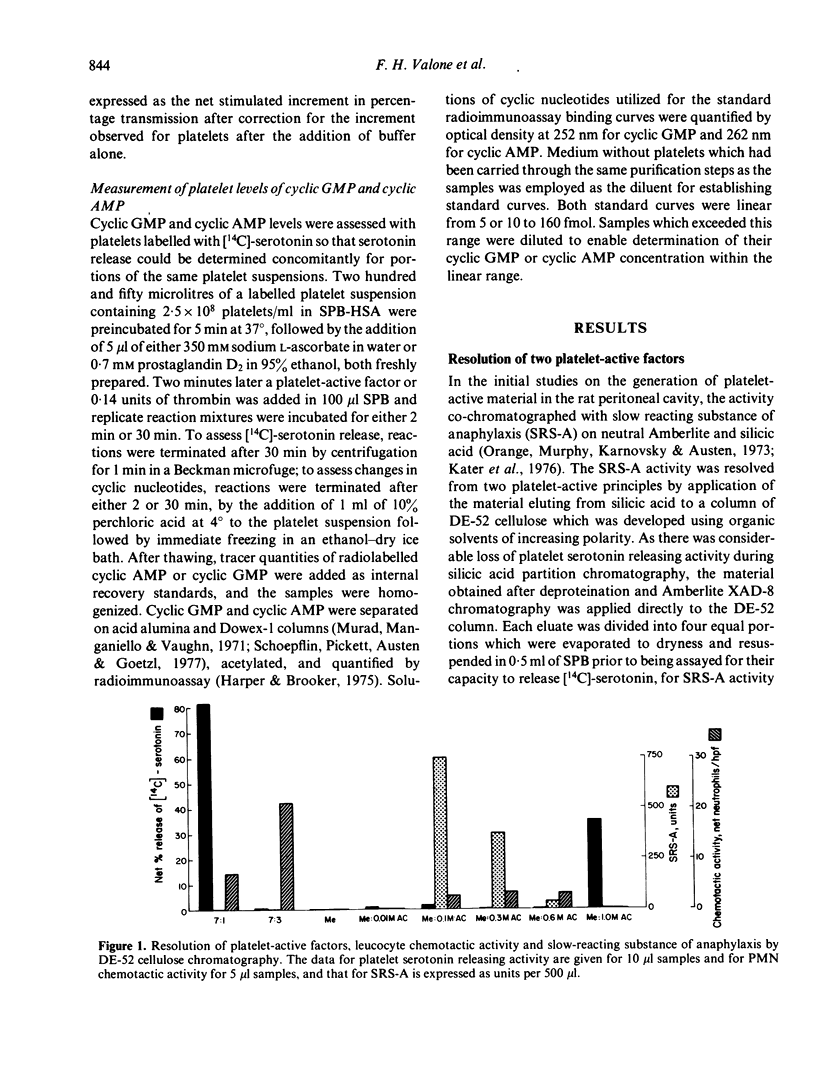
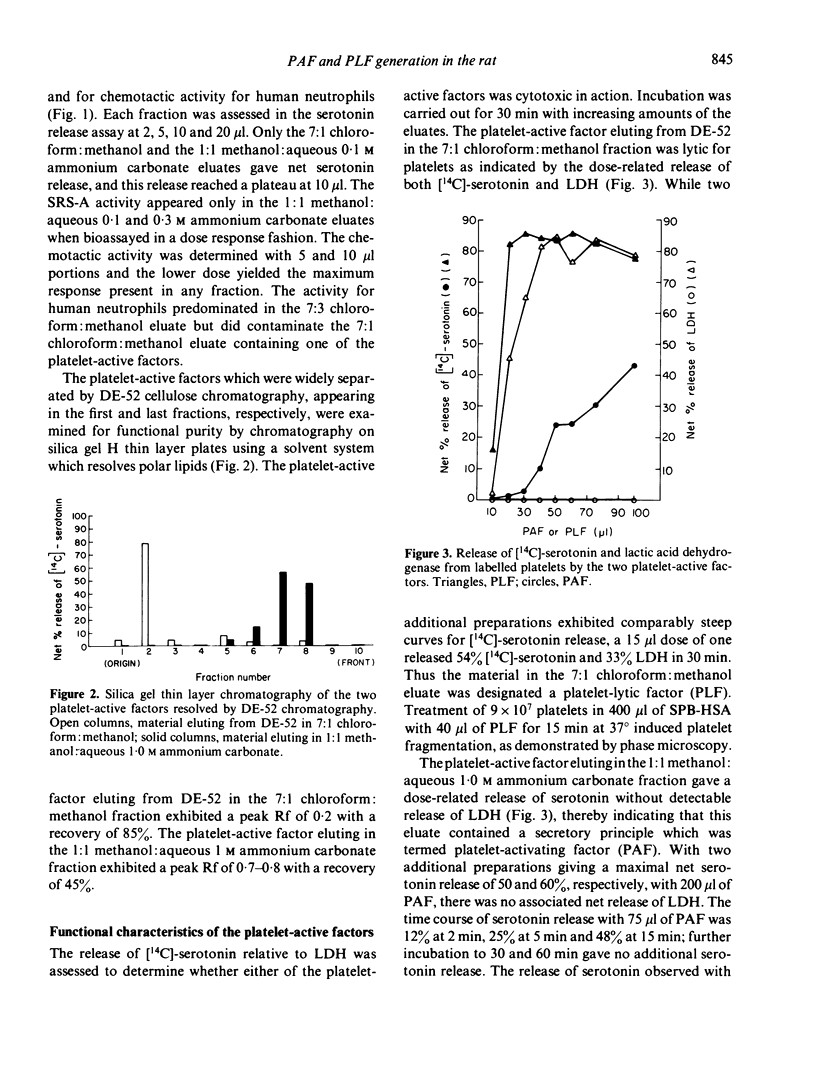
Antigen challenge of the rat peritoneal cavity which had been prepared with IgGa-rich antiserum generated activities which released [14C]-serotonin from pre-labelled human platelets. After adsorption of these activities onto Amberlite XAD-8 and elution in 80% ethanol, two factors of differing polarity were resolved by chromatography on diethylaminoethyl cellulose in organic solvents. The activity eluting in the 7:1 chloroform:methanol solvent contained a platelet-lytic factor (PLF) assessed by the parallel release of lactic acid dehydrogenase and [14C]-serotonin; the cytotoxicity of this fraction was confirmed by phase-contrast microscopy examination which demonstrated fragmentation of the exposed platelets. The activity eluting in the 1:1 methanol: aqueous 1.0 M ammonium carbonate solvent was a platelet-activating factor (PAF) as defined by release of [14C]-serotonin without lactic acid dehydrogenase. Both the lytic and the activating principles were separable from slow reacting substance of anaphylaxis and polymorphonuclear leucocyte chemotactic activity, and each presented a single activity peak of differing mobility when chromatographed on silica gel H plates. Human eosinophil phospholipase D inactivated the lytic factor by more than 85% in 2 h at 37 degrees without affecting the activity of the activating factor. The release of [14C]-serotonin induced by the PAF was not affected by the absence of calcium from the medium or by elevations in the platelet concentrations of cyclic AMP or cyclic GMP that resulted from pre-incubation of platelets with prostaglandin D2 or sodium ascorbate, respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. EFFECTS OF INORGANIC IONS AND OF PLASMA PROTEINS ON THE AGGREGATION OF BLOOD PLATELETS BY ADENOSINE DIPHOSPHATE. J Physiol. 1964 Mar;170:397–414. doi: 10.1113/jphysiol.1964.sp007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Le Couedic J. P., Polonsky J., Tence M. Structural analysis of purified platelet-activating factor by lipases. Nature. 1977 Sep 8;269(5624):170–171. doi: 10.1038/269170a0. [DOI] [PubMed] [Google Scholar]
- Benveniste J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature. 1974 Jun 7;249(457):581–582. doi: 10.1038/249581a0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. doi: 10.1084/jem.131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater L. A., Goetzl E. J., Austen K. F. Isolation of human eosinophil phospholipase D. J Clin Invest. 1976 May;57(5):1173–1180. doi: 10.1172/JCI108385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravis T. C., Henson P. M. IgE-induced release of a platelet-activating factor from rabbit lung. J Immunol. 1975 Dec;115(6):1677–1681. [PubMed] [Google Scholar]
- Long C., Odavić R., Sargent E. J. The action of cabbage-leaf phospholipase D upon lysolecithin. Biochem J. 1967 Jan;102(1):216–220. doi: 10.1042/bj1020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F., Manganiello V., Vaughan M. A simple, sensitive protein-binding assay for guanosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):736–739. doi: 10.1073/pnas.68.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange R. P., Murphy R. C., Karnovsky M. L., Austen K. F. The physicochemical characteristics and purification of slow-reacting substance of anaphylaxis. J Immunol. 1973 Mar;110(3):760–770. [PubMed] [Google Scholar]
- Schoenbechler M. J., Barbaro J. F. The requirement for sensitized lymphocytes in one form of antigen-induced histamine release from rabbit platelets. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1247–1251. doi: 10.1073/pnas.60.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepflin G. S., Pickett W., Austen K. F., Goetzl E. J. Elevation of the cyclic GMP concentration in human platelets by sodium ascorbate and 5-hydroxytryptamine. J Cyclic Nucleotide Res. 1977 Oct;3(5):355–365. [PubMed] [Google Scholar]
- Takahashi H., Webster M. E., Newball H. H. Separation of slow reacting substance of anaphylaxis (SRS-A) from human lung into four biologically active fractions. J Immunol. 1976 Sep;117(3):1039–1044. [PubMed] [Google Scholar]
- Valone F. H., Austen K. F., Goetzl E. J. Modulation of the random migration of human platelets. J Clin Invest. 1974 Nov;54(5):1100–1106. doi: 10.1172/JCI107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone F. H., Goetzl E. J. Immunologic release in the rat peritoneal cavity lipid chemotactic and chemokinetic factors for polymorphonuclear leukocytes. J Immunol. 1978 Jan;120(1):102–108. [PubMed] [Google Scholar]


