Abstract
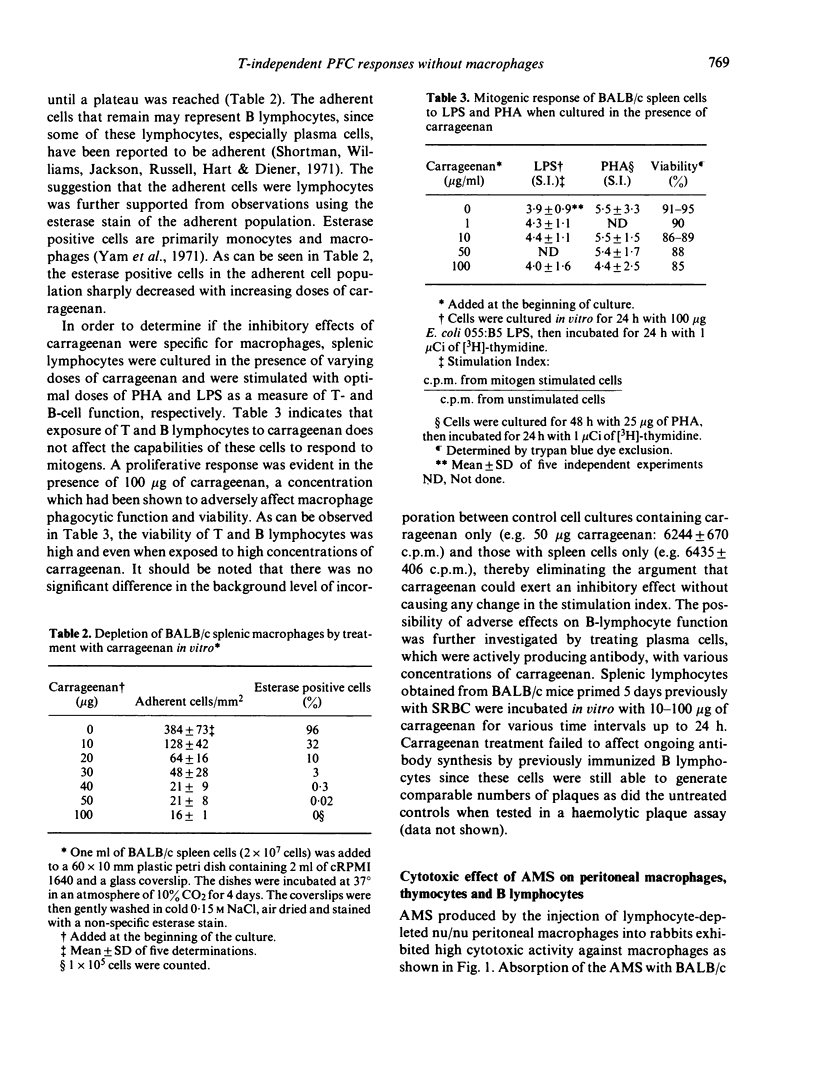
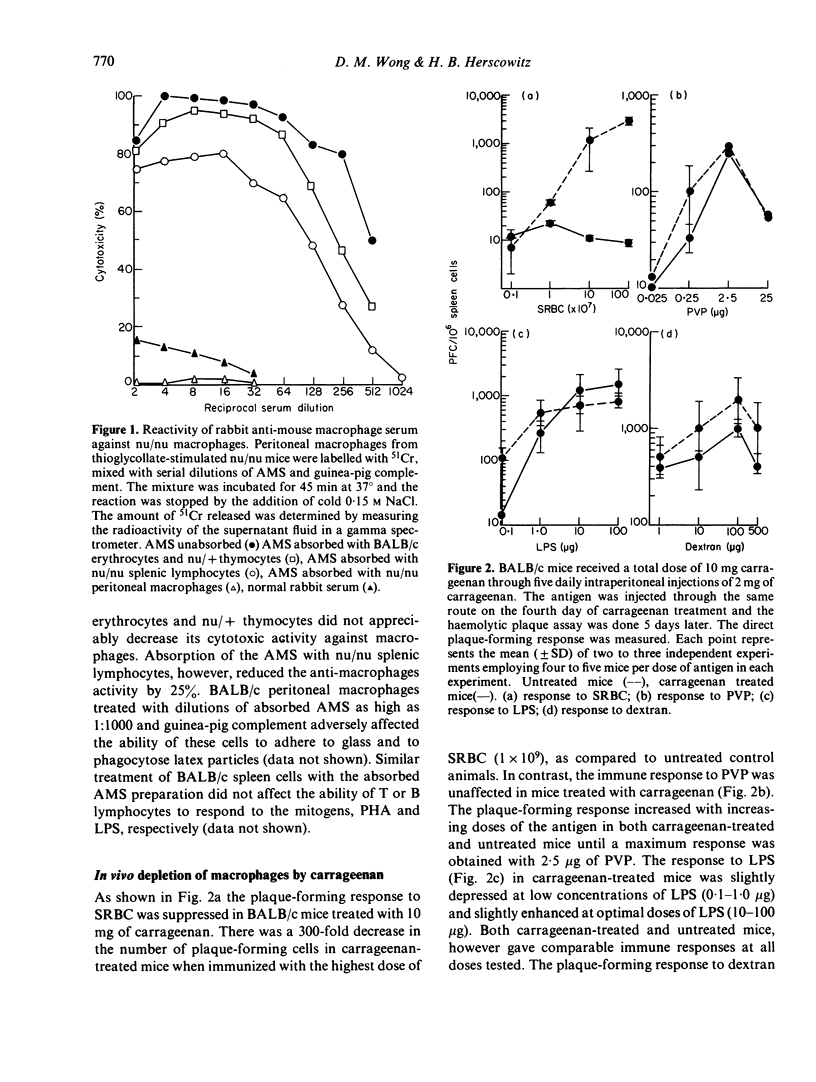
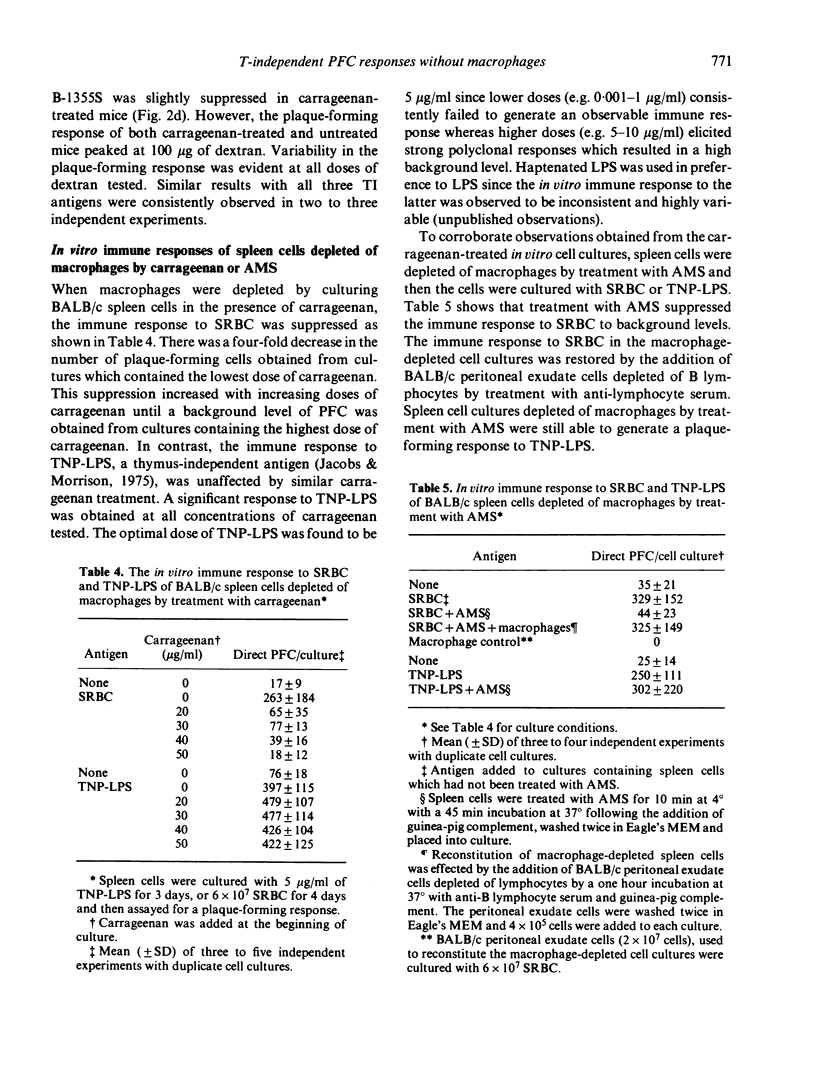
Carrageenan, a sulphated polysaccharide, and rabbit anti-mouse macrophage serum, were used to inhibit macrophage function in BALB/c mice as well as to deplete macrophages from spleen cell cultures in an attempt to determine the requirement for macrophages in the immune response to several thymus-independent antigens. Carrageenan inhibited macrophage function and was cytotoxic at low concentrations. The ability of T and B lymphocytes to undergo mitogen-induced proliferation in the presence of PHA and PLS, respectively, was not affected by in vitro exposure of lymphoid cells to carrageenan. BALB/c mice injected with carrageenan demonstrated a suppressed immune response to SRBC, a thymus-dependent antigen, but not to E. coli LPS, polyvinyl-pyrrolidone or dextran B-1355S, all of which are known to be thymus independent antigens. The sensitivity of the in vivo immune response to SRBC after depletion of macrophages by carrageenan treatment was confirmed in vitro using the Marbrook--Diener culture system. The in vitro immune response to TNP-LPS was unaffected by either carrageenan treatment or treatment of BALB/c spleen cells with AMS and complement. The results of experiments which utilized the two anti-macrophage reagents, carrageenan and AMS, both in vivo and in vitro systems, suggest that the immune response to thymus-independent antigens does not require the participation of macrophages.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice D. E., Gruwell D. G., Salvaggio J. E., Hoffmann E. O. Suppression of primary immunization by carrageenan - a macrophage toxic agent. Immunol Commun. 1972;1(6):615–625. doi: 10.3109/08820137209022968. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Schwartz H. J., Graham R. C., Jr Spectrum and possible mechanism of carrageenan cytotoxicity. Am J Pathol. 1971 Aug;64(2):387–404. [PMC free article] [PubMed] [Google Scholar]
- Chaouat G., Howard J. G. Influence of reticuloendothelial blockade on the induction of tolerance and immunity by polysaccharides. Immunology. 1976 Feb;30(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- Chused T. M., Kassan S. S., Mosier D. E. Macrophage requirement for the in vitro response to TNP Ficoll: a thymic independent antigen. J Immunol. 1976 Jun;116(6):1579–1581. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Ghanta V. K., Hamlin N. M., Pretlow T. G., Hiramoto R. N. Use of dextran-conjugated SRBC for the detection of MOPC 104E tumor cells. J Immunol. 1972 Oct;109(4):810–815. [PubMed] [Google Scholar]
- Glode L. M., Rosenstreich D. L. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J Immunol. 1976 Dec;117(6):2061–2066. [PubMed] [Google Scholar]
- Hirsch M. S., Gary G. W., Jr, Murphy F. A. In vitro and in vivo properties of antimacrophage sera. J Immunol. 1969 Mar;102(3):656–661. [PubMed] [Google Scholar]
- Ishizaka S., Otani S., Morisawa S. Effects of carrageenan on immune responses. Studies on the macrophage dependency of various antigens after treatment with carrageenan. J Immunol. 1977 Apr;118(4):1213–1218. [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Stimulation of a T-independent primary anti-hapten response in vitro by TNP-lipopolysaccharide (TNP-LPS). J Immunol. 1975 Jan;114(1 Pt 2):360–364. [PubMed] [Google Scholar]
- Lemke H., Coutinho A., Opitz H. G., Gronowicz E. Macrophages suppress direct B-cell activation by lipopolysaccharide. Scand J Immunol. 1975;4(7):707–720. doi: 10.1111/j.1365-3083.1975.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Marbrook J. Primary immune response in cultures of spleen cells. Lancet. 1967 Dec 16;2(7529):1279–1281. doi: 10.1016/s0140-6736(67)90393-5. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Nelson D. S. Production by stimulated macrophages of factors depressing lymphocyte transformation. Nature. 1973 Nov 30;246(5431):306–307. doi: 10.1038/246306a0. [DOI] [PubMed] [Google Scholar]
- Poe W. J., Michael J. G. The lack of cellular cooperation in the immune response to E. coli 0127. J Immunol. 1974 Sep;113(3):1033–1038. [PubMed] [Google Scholar]
- Proctor M. L., Herscowitz H. B. Preparation and properties of an antiplasma cell serum directed against a defined plasmacytoma cell population. J Immunol. 1975 Dec;115(6):1642–1649. [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Rumjanek V. M., Watson S. R., Sljivić V. S. A re-evaluation of the role of macrophages in carrageenan-induced immunosuppression. Immunology. 1977 Sep;33(3):423–432. [PMC free article] [PubMed] [Google Scholar]
- SMITH D. B., COOK W. H., NEAL J. L. Physical studies on carrageenin and carragenin fractions. Arch Biochem Biophys. 1954 Nov;53(1):192–204. doi: 10.1016/0003-9861(54)90246-5. [DOI] [PubMed] [Google Scholar]
- Shortman K., Byrd W., Williams N., Brunner K. T., Cerottini J. C. The separation of different cell classes from lymphoid organs. The relationship between the adherence properties and the buoyant density of sub-populations of "B" and "T" lymphocytes. Aust J Exp Biol Med Sci. 1972 Jun;50(3):323–336. doi: 10.1038/icb.1972.26. [DOI] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Williams N., Jackson H., Russell P., Byrt P., Diener E. The separation of different cell classes from lymphoid organs. IV. The separation of lymphocytes from phagocytes on glass bead columns, and its effect on subpopulations of lymphocytes and antibody-forming cells. J Cell Biol. 1971 Mar;48(3):566–579. doi: 10.1083/jcb.48.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Kaplan A. M., Morahan P. S. Identification of a macrophage-specific cell surface antigen. J Immunol. 1976 Feb;116(2):273–278. [PubMed] [Google Scholar]
- Thomson A. W., Wilson A. R., Cruickshank W. J., Jeffreis A. H. Evidence for a selective cytotoxic effect of carrageenan on cells of the immune system in vivo and in vitro. Experientia. 1976 Apr 15;32(4):525–526. doi: 10.1007/BF01920837. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wilton J. M., Rosenstreich D. L., Oppenheim J. J. The role of macrophages in the production of lymphokines by T and B lymphocytes. J Immunol. 1975 Apr;114(4):1296–1301. [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages in the induction and expression of acquired immunity. J Reticuloendothel Soc. 1976 Jul;20(1):57–65. [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages: subclasses of peritoneal macrophages with different antigen-binding activities and immune complex receptors. Immunology. 1974 May;26(5):1025–1037. [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yoshinaga M., Yoshinaga A., Waksman B. H. Regulation of lymphocyte responses in vitro. I. Regulatory effect of macrophages and thymus-dependent (T) cells on the response of thymus-independent (B) lymphocytes to endotoxin. J Exp Med. 1972 Oct 1;136(4):956–961. doi: 10.1084/jem.136.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]


