Abstract
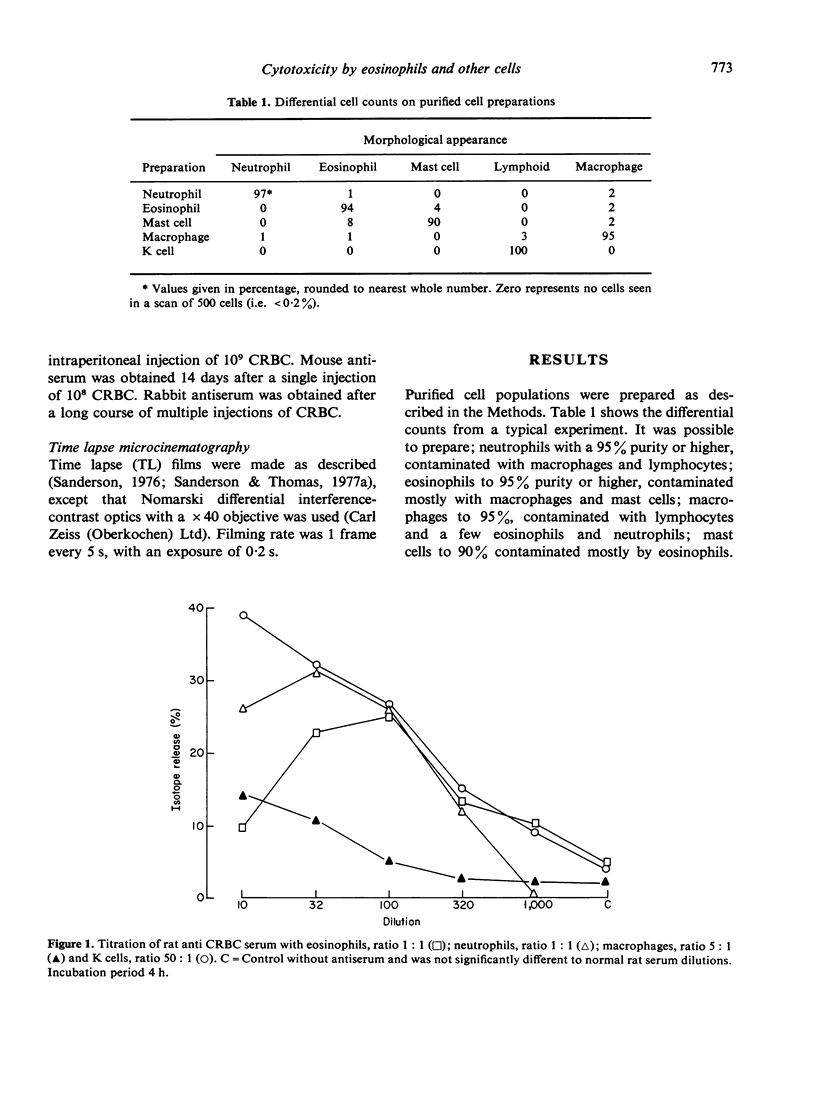
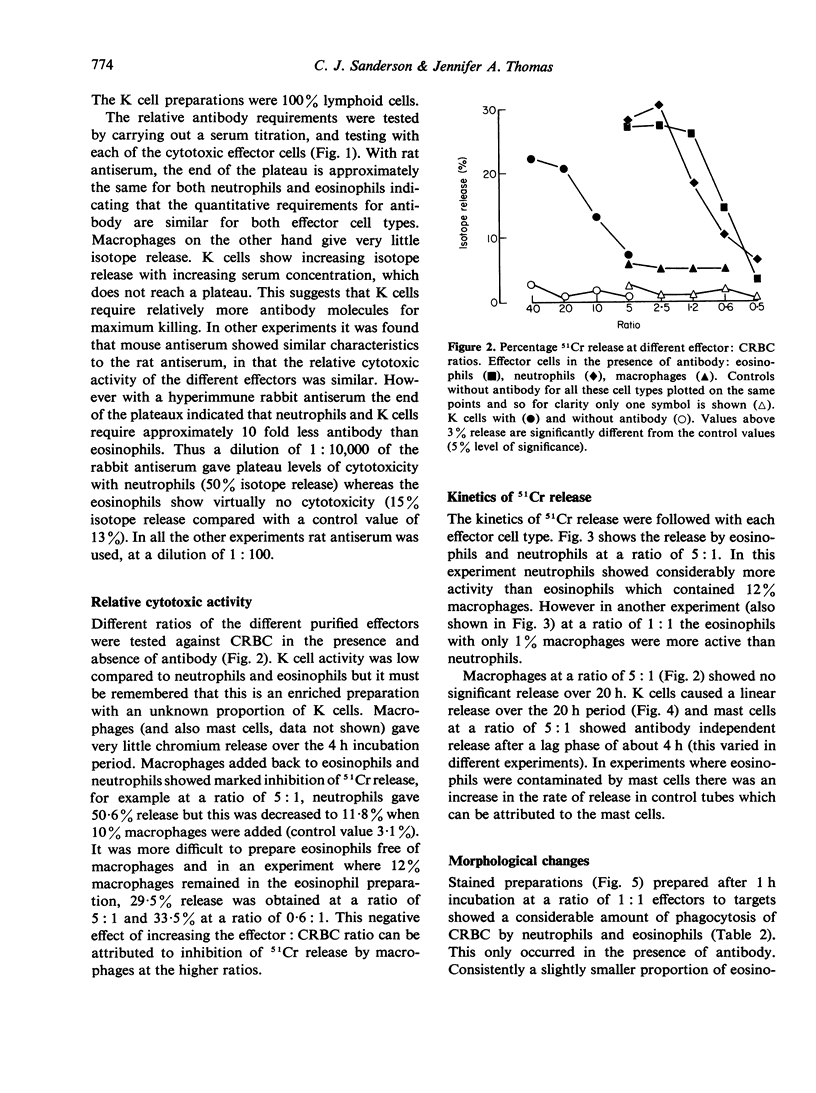
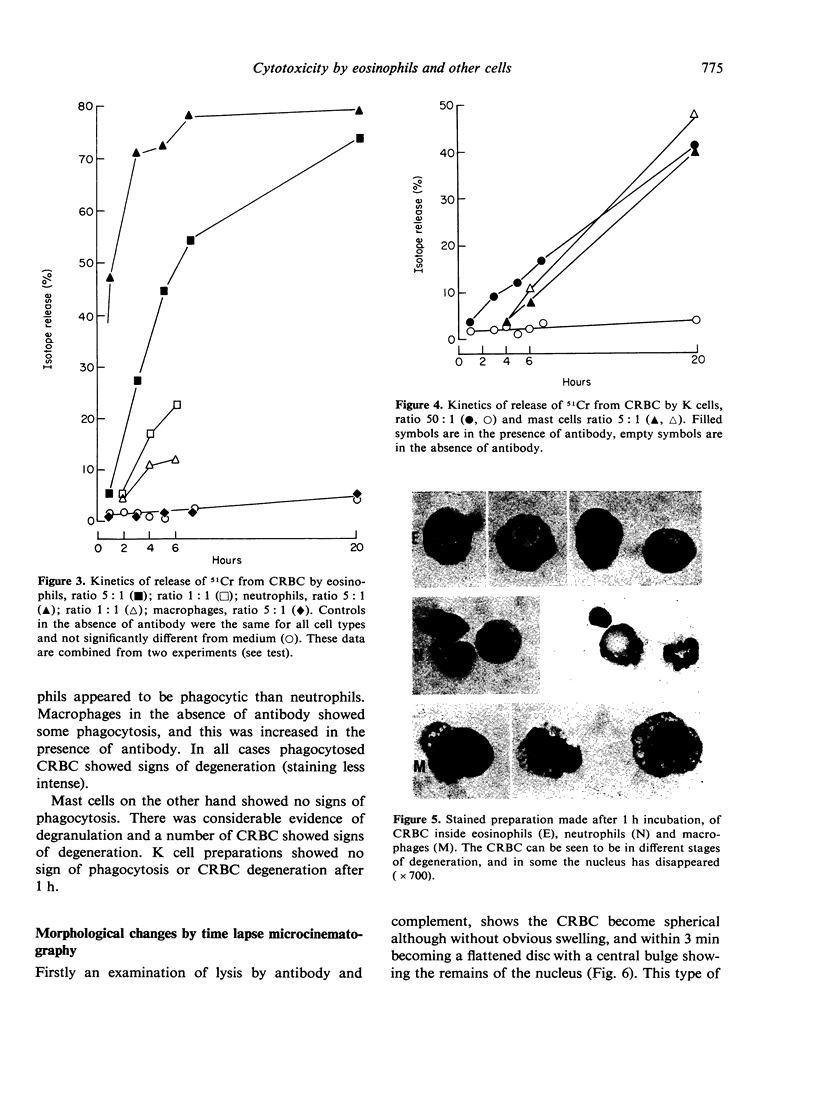
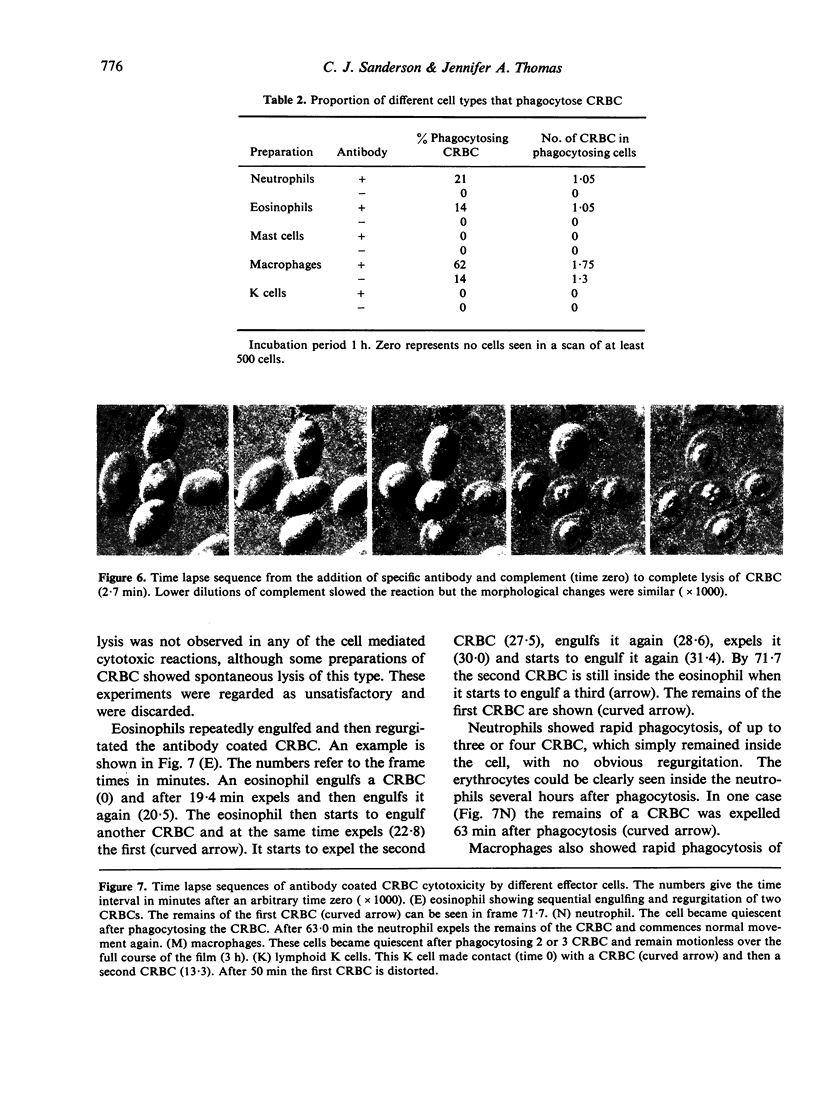
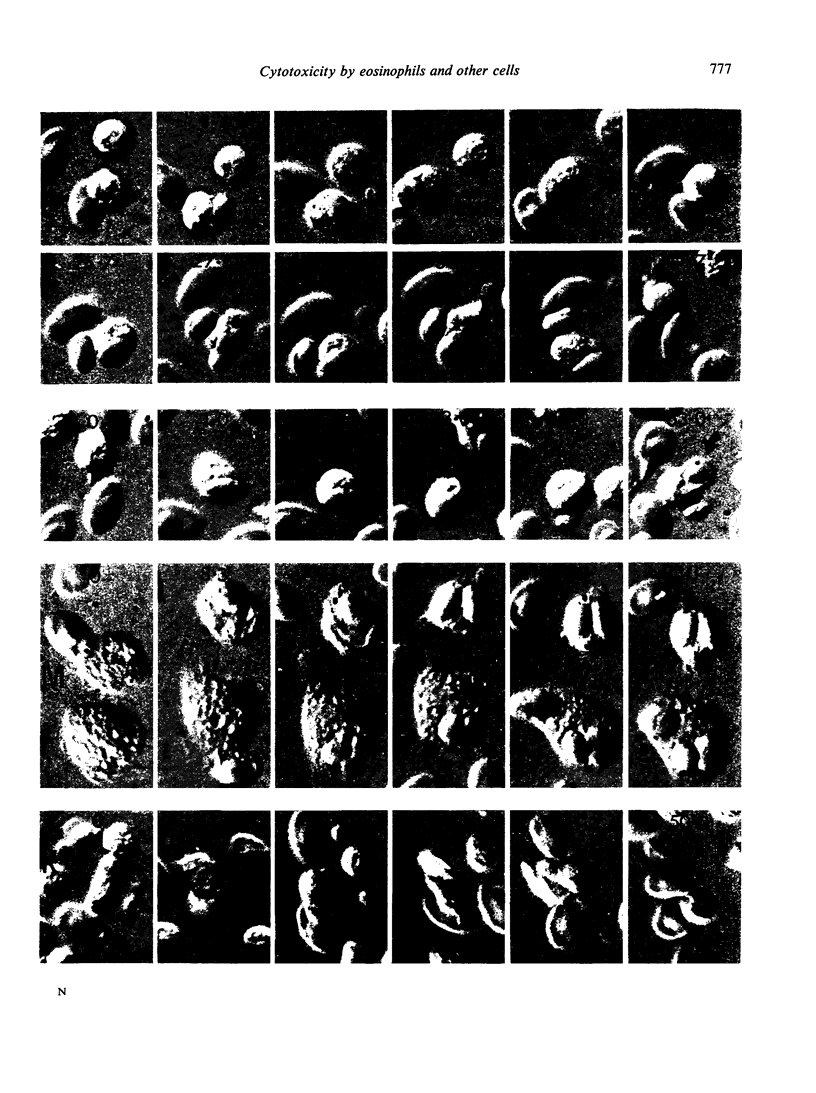
Antibody dependent cytotoxicity of chicken erythrocytes by purified rat eosinophils, neutrophils, macrophages and K cells has been compared by 51Cr release and time lapse microcinematography. Techniques have been developed for purifying these effector cell types. Both eosinophils and neutrophils cause rapid release of 51Cr from erythrocytes. Time lapse observations indicated that this was the result of phagocytosis. Eosinophils show rapid membrane movement and repeatedly engulf and regurgitate the erythrocytes. On the other hand, neutrophils become quiescent after phagocytosing erythrocytes, and remain quiescent until the remains of the cell are expelled. Neutrophils presumably have a mechanism for the release of soluble material, as 51Cr is released rapidly. Macrophages show a similar quiescence after phagocytosis, but in these cells there is apparently no rapid mechanism to expel material, as there is no significant 51Cr release over 20 h. K cells appear to damage chicken erythrocytes more slowly than they destroy tumour cells. Mast cells cause antibody-independent cytotoxicity which can be attributed to the release of toxic materials. None of these effector cells produced the type of lysis seen with antibody and complement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Lake P., Sabbadini E., Sehon A. H. Specificity of cell-mediated transplantation reactions. I. Studies with the technique of inhibition of migration and an assay of tumour immunity in vivo. Immunology. 1974 Sep;27(3):427–439. [PMC free article] [PubMed] [Google Scholar]
- Penfold P. L., Greenberg A. H., Roitt I. M. Characteristics of the effector cells mediating cytotoxicity against antibody-coated target cells. III. Ultrastructural studies. Clin Exp Immunol. 1976 Jan;23(1):91–97. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Clark I. A., Taylor G. A. Different effector cell types in antibody-dependent cell-mediated cytotoxicity. Nature. 1975 Jan 31;253(5490):376–377. doi: 10.1038/253376a0. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Lopez A. F., Moreno M. M. Eosinophils and not lymphoid K cells kill Trypanosoma cruzi epimastigotes. Nature. 1977 Jul 28;268(5618):340–341. doi: 10.1038/268340a0. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Taylor G. A. Antibody-dependent cell-mediated cytotoxicity in the rat. The role of macrophages. Immunology. 1976 Jan;30(1):117–121. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J. The mechanism of T cell mediated cytotoxicity. II. Morphological studies of cell death by time-lapse microcinematography. Proc R Soc Lond B Biol Sci. 1976 Jan 20;192(1107):241–255. doi: 10.1098/rspb.1976.0011. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A. The mechanism of K cell (antibody-dependent) cell mediated cytotoxicity. I. The release of different cell components. Proc R Soc Lond B Biol Sci. 1977 Jul 20;197(1129):407–415. doi: 10.1098/rspb.1977.0077. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A. The mechanism of K cell (antibody-dependent) cell mediated cytotoxicity. II. Characteristics of the effector cell and morphological changes in the target cell. Proc R Soc Lond B Biol Sci. 1977 Jul 20;197(1129):417–424. doi: 10.1098/rspb.1977.0078. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Schur P. H. Lectin-dependent neutrophil-mediated cytotoxicity. I. Characteristics. Immunology. 1976 Aug;31(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. Studies on blood eosinophils. I. Patients with a transient eosinophilia. Clin Exp Immunol. 1976 Jun;24(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Thomas W. R., Holt P. G., Keast D. Phagocytosis and processing of bacteria by peritoneal macrophages. J Reticuloendothel Soc. 1974 Jan;15(1):16–21. [PubMed] [Google Scholar]