Abstract
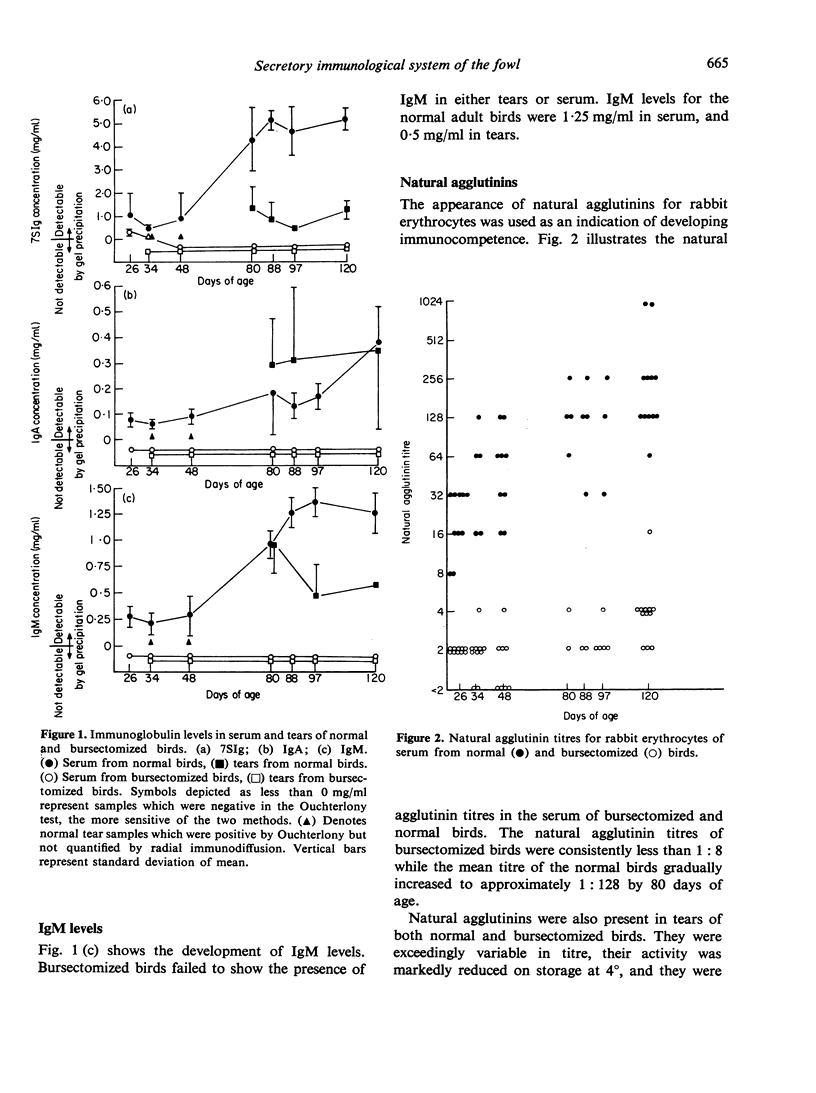
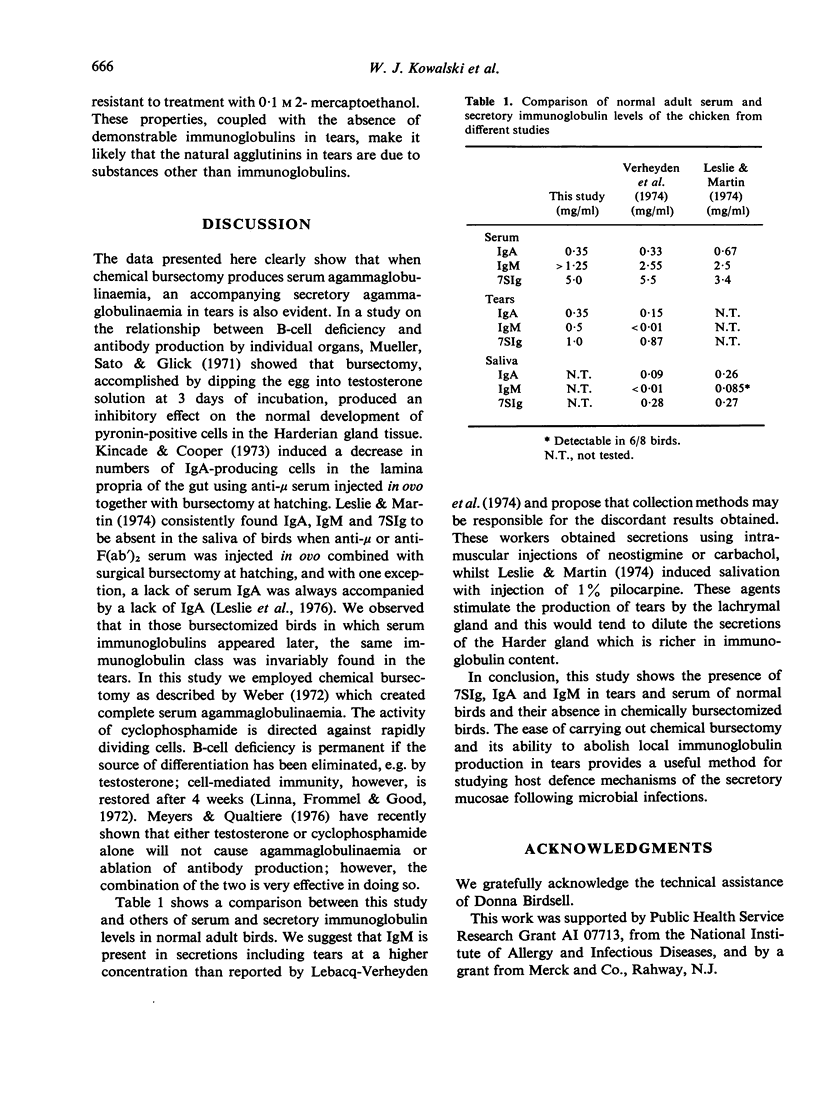
The effect of chemical bursectomy (3-5 mg testosterone cypionate injected at 12 days in ovo followed by 4-0 mg cyclophosphamide intraperitoneally at days 1 and 2 post-hatching) on concentration of immunoglobulins in tears and serum of chickens was investigated. Tears and serum samples were collected from bursectomized and normal control birds between days 26 and 120 post-hatching and were assayed for IgA, 7SIg and IgM concentrations using double gel-diffusion and radial immunodiffusion. No immunoglobulins other than passively acquired 7SIg were detected in the sera or tears of 23 out of 30 bursectomized birds throughout the time period. Sera were also assayed for natural agglutinins; normal birds had titres for rabbit erythrocytes which gradually increased from a mean of 1:24 at day 26 to 1:280 at day 120, while bursectomized chickens had titres of less than 1:8 throughout the experimental period. It was concluded that chemical bursectomy effectively abolished the secretory immunoglobulins normally present in tears as well as serum immunoglobulins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini B., Wick G., Rose E., Orlans E. Immunoglobulin production in chicken Harderian glands. Int Arch Allergy Appl Immunol. 1974;47(1):23–34. doi: 10.1159/000231198. [DOI] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B. Localized lymphoid tissues and plasma cells in paraocular and paranasal organ systems in chickens. Am J Pathol. 1968 Nov;53(5):735–751. [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Gauldie J., Perey D. Y. Synthesis of IgG, IgA, IgM by chicken tissues: immunofluorescent and 14C amino acid incorporation studies. J Immunol. 1973 Oct;111(4):1112–1118. [PubMed] [Google Scholar]
- Cho H. C., Kramer T. T. Radial immunodiffusion of chicken serum proteins. I. Standardization of optimal test procedures. Can J Comp Med. 1970 Oct;34(4):341–346. [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Immunoglobulin A: site and sequence of expression in developing chicks. Science. 1973 Jan 26;179(4071):398–400. doi: 10.1126/science.179.4071.398. [DOI] [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Vaerman J. P., Heremans J. F. Quantification and distribution of chicken immunoglobulins IgA, IgM and IgG in serum and secretions. Immunology. 1974 Oct;27(4):683–692. [PMC free article] [PubMed] [Google Scholar]
- Leslie G. A., Martin L. N. Studies on the secretory immunologic system of fowl. 3. Serum and secretory IgA of the chicken. J Immunol. 1973 Jan;110(1):1–9. [PubMed] [Google Scholar]
- Leslie G. A., Martin L. N. The secretory immunologic system of fowl. IV. Serum and salivary immunoglobulins in normal, agammaglobulinemic and dysgammaglobulinemic chickens. Int Arch Allergy Appl Immunol. 1974;46(6):834–841. [PubMed] [Google Scholar]
- Leslie G. A., Stankus R. P., Martin L. N. Secretory immunological system of fowl. V. The gallbladder: an integral part of the secretory immunological system of fowl. Int Arch Allergy Appl Immunol. 1976;51(2):175–185. doi: 10.1159/000231590. [DOI] [PubMed] [Google Scholar]
- Linna T. J., Frommel D., Good R. A. Effects of early cyclophosphamide treatment on the development of lymphoid organs and immunological functions in the chickens. Int Arch Allergy Appl Immunol. 1972;42(1):20–39. doi: 10.1159/000230590. [DOI] [PubMed] [Google Scholar]
- Martin L. N., Leslie G. A. Ontogeny of IgA in normal and neonatally bursectomized chickens, with corroborative data on IgY and IgM. Proc Soc Exp Biol Med. 1973 May;143(1):241–243. doi: 10.3181/00379727-143-37294. [DOI] [PubMed] [Google Scholar]
- Meyers P., Qualtiere L. F. The efficacy of chemical bursectomy in chickens with congenital leukosis-virus infection. Immunology. 1976 Oct;31(4):527–532. [PMC free article] [PubMed] [Google Scholar]
- Mueller A. P., Sato K., Glick B. The chicken lacrimal gland, gland of Harder, caecal tonsil, and accessory spleens as sources of antibody-producing cells. Cell Immunol. 1971 Apr;2(2):140–152. doi: 10.1016/0008-8749(71)90033-5. [DOI] [PubMed] [Google Scholar]
- Seto F., Henderson W. G. Natural and immune hemagglutinin forming capacity of immature chickens. J Exp Zool. 1968 Dec;169(4):501–511. doi: 10.1002/jez.1401690412. [DOI] [PubMed] [Google Scholar]
- Sundick R. S., Albini B., Wick G. Chicken Harder's gland: evidence for a relatively pure bursa-dependent lymphoid cell population. Cell Immunol. 1973 May;7(2):332–335. doi: 10.1016/0008-8749(73)90256-6. [DOI] [PubMed] [Google Scholar]
- Weber W. T. Proliferative and functional capacity of bursal lymphocytes after transfer to agammaglobulinemic chicks. Cell Immunol. 1972 May;4(1):51–65. doi: 10.1016/0008-8749(72)90005-6. [DOI] [PubMed] [Google Scholar]


