Abstract
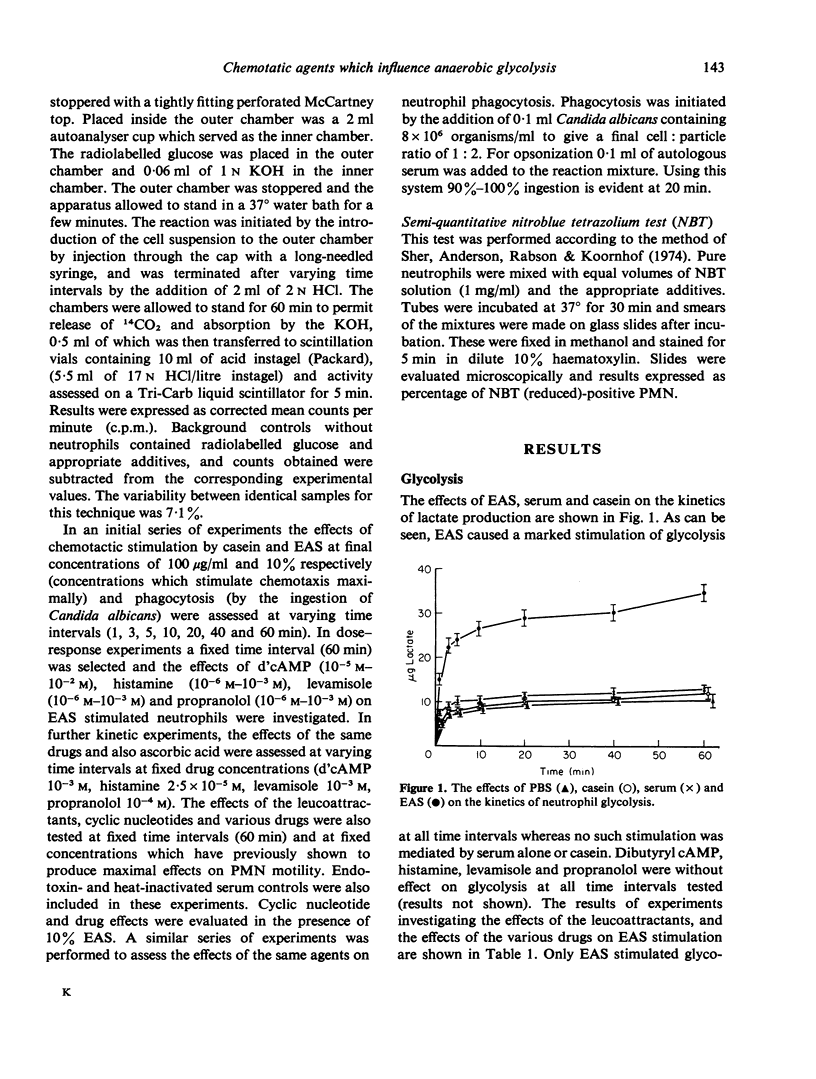
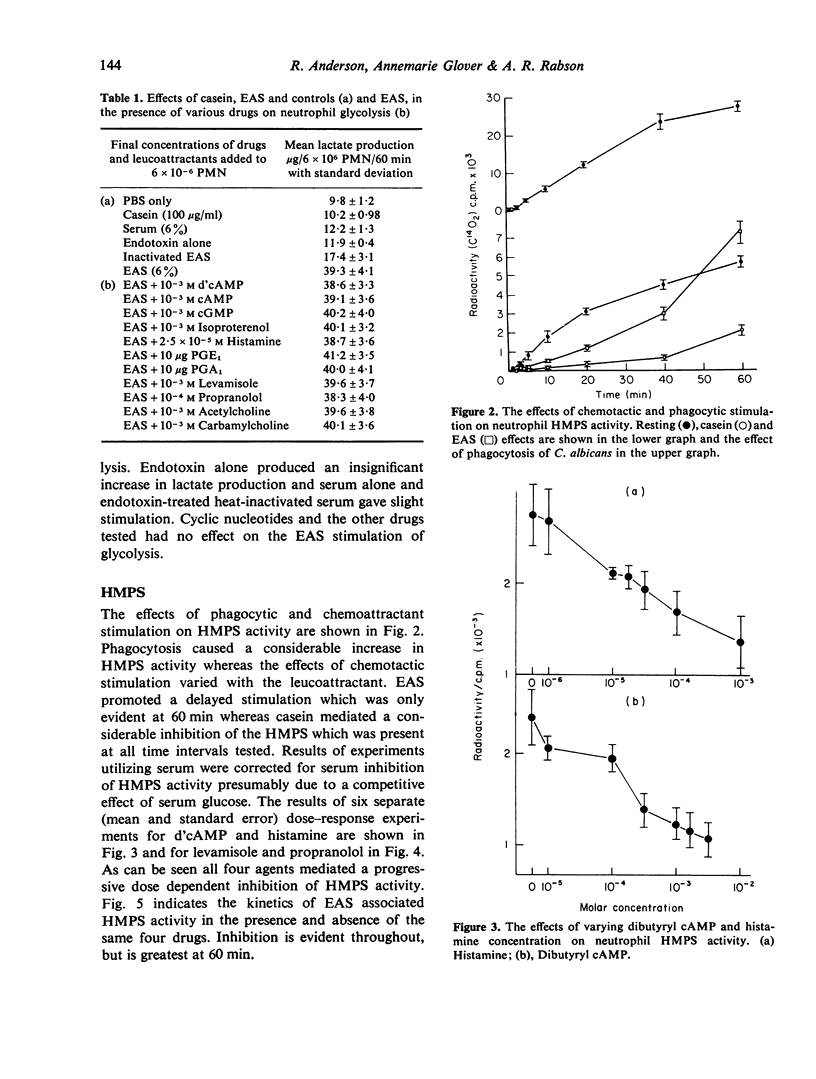
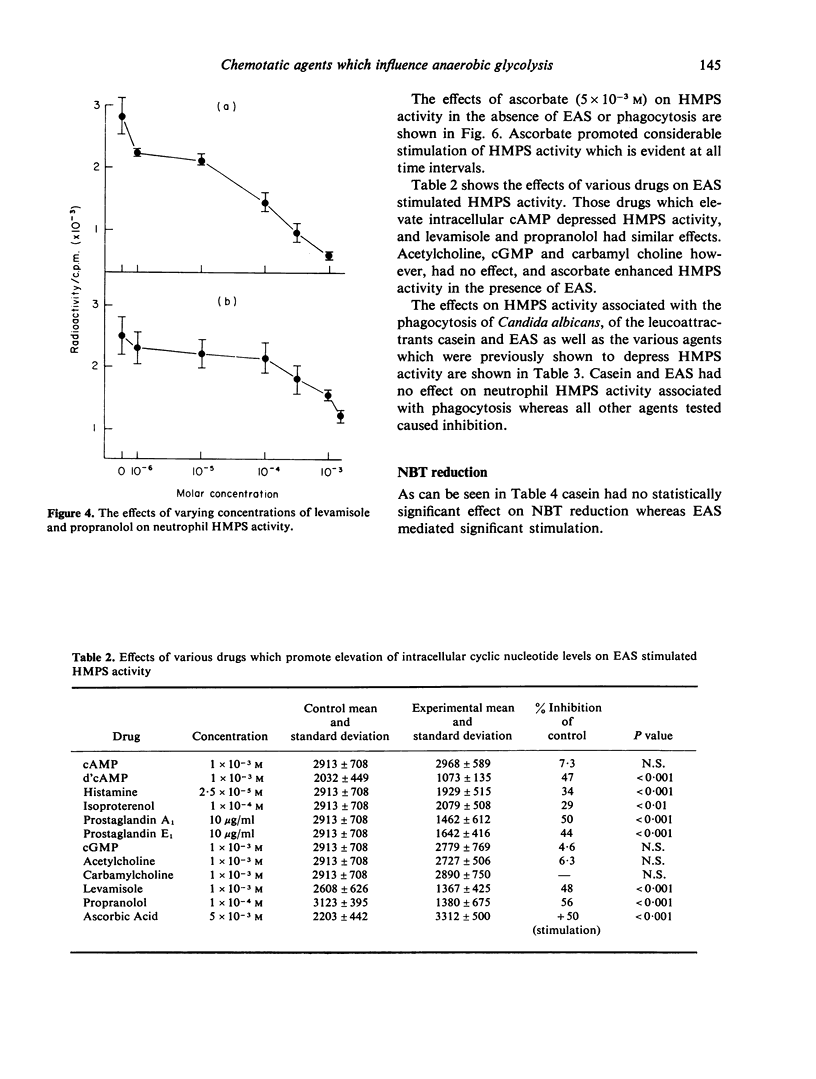
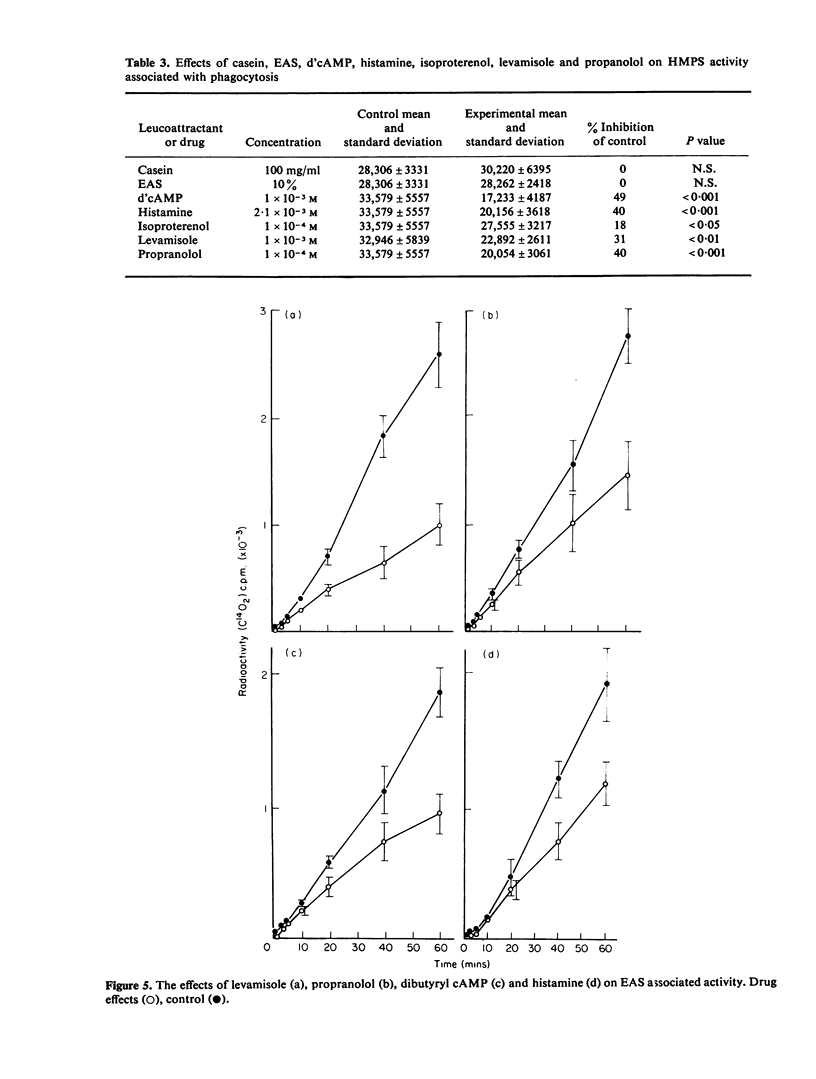
The effects of two chemotactic factors, endotoxin activated serm (EAS) and casein and a number of drugs known to affect intracellular cyclic nucleotide levels and various froms of neutrophil movement, on neutrophil anaerobic glycolysis and hexose monophosphate shunt (HMPS) activity were assessed. EAS caused stimulation of glycolysis. HMPS activity and NBT reduction, but casein was without effect on glycolysis and NBT reduction and inhibited HMPS activity. Drug known to increase intracellular cAMP levels caused a depression of HMPS activity whereas those reported to elevate cGMP had a variety of effects. Glycolysis was not affected by any of these agents. These results indicate a lack of relationship between cyclic nucleotide effect on cell motility and neutrophil glycolysis and HMPS activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Glover A., Koornhof H. J., Rabson A. R. In vitro stimulation of neutrophil motility by levamisole: maintenance of cgmp levels in chemotactically stimulated levamisole-treated neutrophils. J Immunol. 1976 Aug;117(2):428–432. [PubMed] [Google Scholar]
- Anderson R., Glover A., Rabson A. R. The in vitro effects of histamine and metiamide on neutrophil motility and their relationship to intracellular cyclic nucleotide levels. J Immunol. 1977 May;118(5):1690–1696. [PubMed] [Google Scholar]
- Carruthers B. M. Leukocyte motility. I. Method of study, normal variation, effect of physical alterations in environment, and effect of iodoacetate. Can J Physiol Pharmacol. 1966 May;44(3):475–485. doi: 10.1139/y66-056. [DOI] [PubMed] [Google Scholar]
- Carruthers B. M. Leukocyte motility. II. Effect of absence of glucose in medium; effect of presence of deoxyglucose, dinitrophenol, puromycin, actinomycin D, and trypsin on the response to chemotactic substance; effect of segregation of cells from chemotactic substance. Can J Physiol Pharmacol. 1967 Mar;45(2):269–280. doi: 10.1139/y67-029. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R., McCall C. E. Stimulation of the hexose monophosphate shunt in human neutrophils by ascorbic acid: mechanism of action. Antimicrob Agents Chemother. 1972 Jan;1(1):12–16. doi: 10.1128/aac.1.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Hill H. R., Quie P. G., Gogan N., Goldberg N. D. Cyclic GMP and cell movement. Nature. 1973 Oct 26;245(5426):458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Flyer R. H., Finch S. C. The effects of prostaglandin E1 on human granulocyte metabolism during phagocytosis. J Reticuloendothel Soc. 1973 Oct;14(4):325–331. [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Gigli I., Wasserman S., Austen K. F. A neutrophil immobilizing factor derived from human leukocytes. II. Specificity of action on polymorphonuclear leukocyte mobility. J Immunol. 1973 Sep;111(3):938–945. [PubMed] [Google Scholar]
- Goetzl E. J., Wasserman S. I., Gigli I., Austen K. F. Enhancement of random migration and chemotactic response of human leukocytes by ascorbic acid. J Clin Invest. 1974 Mar;53(3):813–818. doi: 10.1172/JCI107620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Feit F., Weissmann G. Enhancement of nitroblue tetrazolium dye reduction by leukocytes exposed to a component of complement in the absence of phagocytosis. J Immunol. 1975 Jan;114(1 Pt 2):516–518. [PubMed] [Google Scholar]
- Qualliotine D., DeChatelet L. R., McCall C. E., Cooper M. R. Stimulation of oxidative metabolism in polymorphonuclear leukocytes by catecholamines. J Reticuloendothel Soc. 1972 Mar;11(3):263–276. [PubMed] [Google Scholar]
- Rabson A. R., Anderson R., Lomnitzer R., Koornhof H. J. In vitro effects of prostaglandins on polymorphonuclear leucocyte function. S Afr Med J. 1974 Oct 16;0(0 Suppl):44–50. [PubMed] [Google Scholar]
- Sher R., Anderson R., Rabson A. R., Koornhof H. J. Standardisation of the nitro-blue tetrazolium test and factors affecting its clinical application. S Afr Med J. 1974 Feb 9;48(6):209–212. [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]


