Abstract
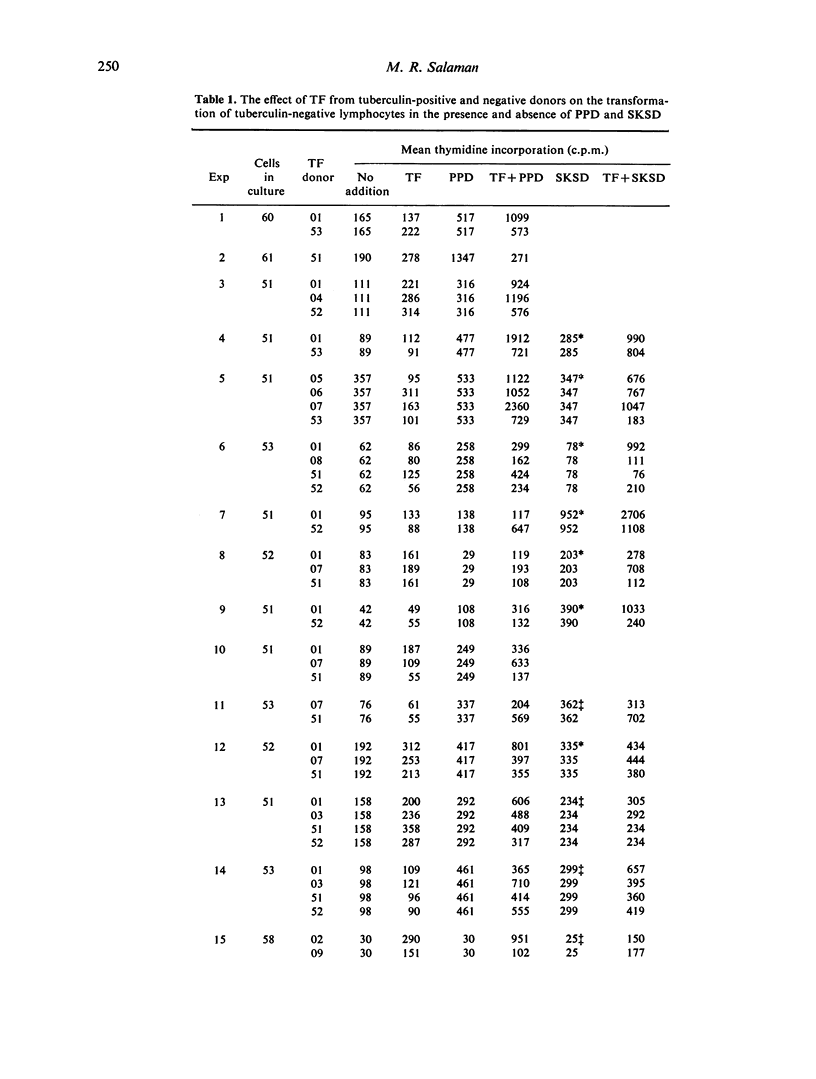
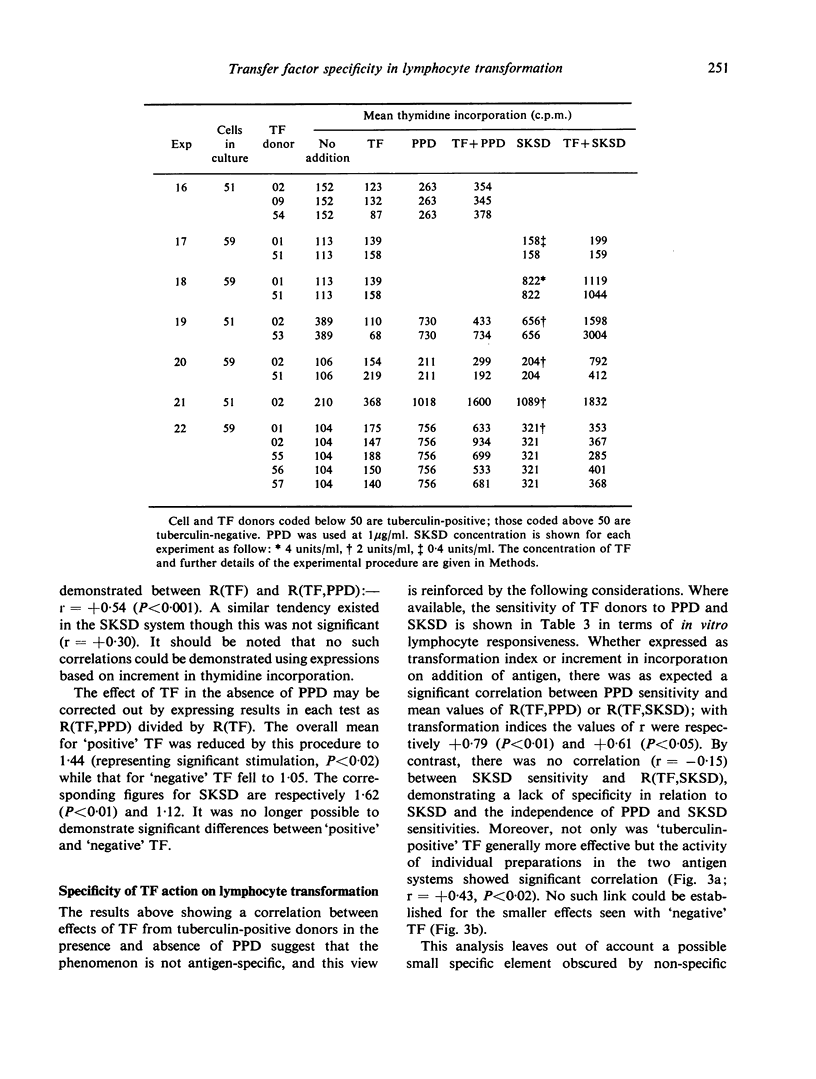
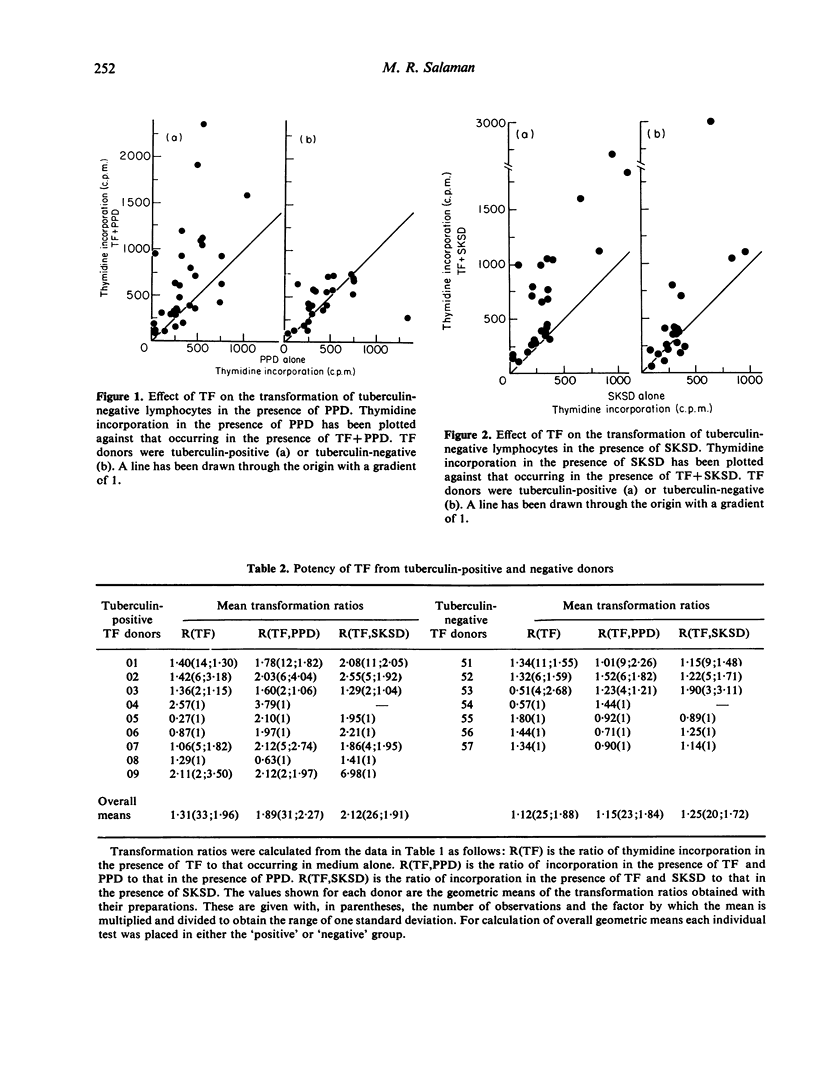
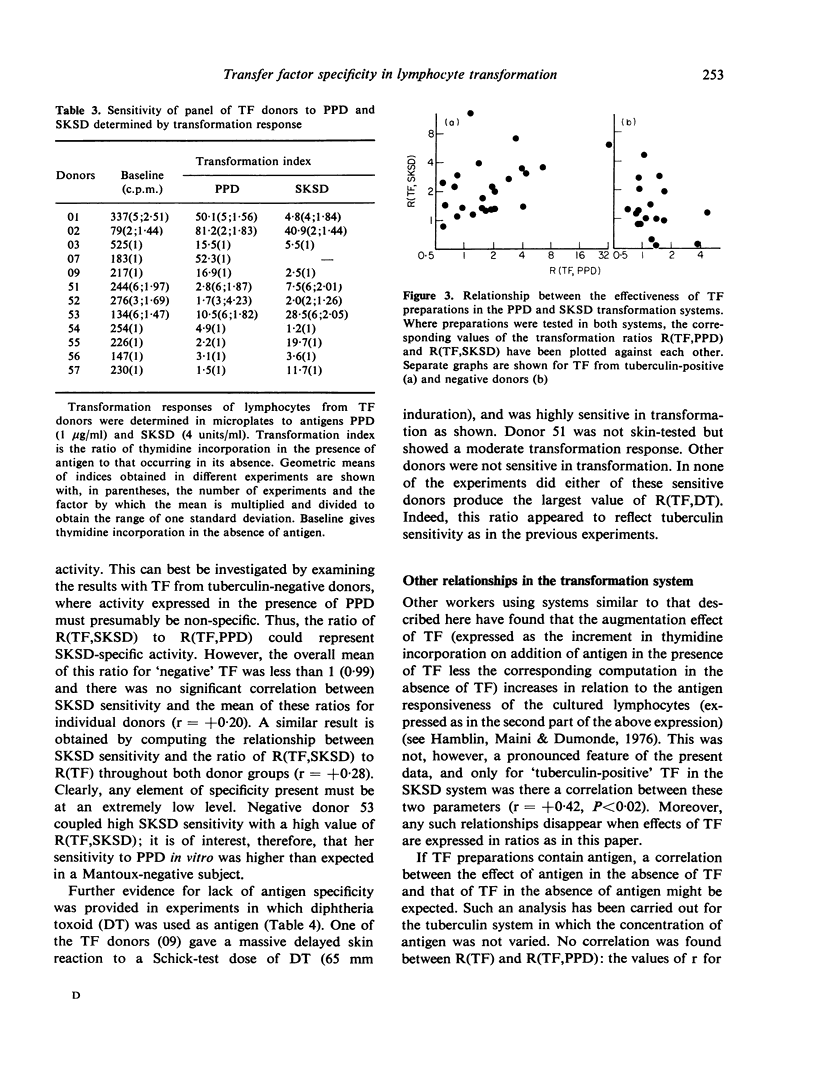
Dialysable transfer factor (TF) was prepared from the buffy-coat cells of donors with known cell-mediated reactivity to tuberculin (PPD), streptococcal protein (SKSD) and diphtheria toxoid (DT). The effect of such preparations on the transformation by these antigens of lymphocytes from tuberculin-negative donors was investigated. Transformation was determined as incorporation of tritiated thymidine. The concentrations of SKSD and DT were adjusted for different lymphocyte donors so as to give, in the absence of TF, a low index of transformation (less than 10-fold) comparable to that obtained with PPD. TF from tuberculin-positive donors stimulated antigen-induced transformation by on average approximately 2-fold whereas TF from tuberculin-negative donors generally had little effect. This was so not for PPD as antigen but also for SKSD and DT, and sensitivity of TF donor to SKSD of DT was not a determining factor. TF also frequently increased background transformation in the absence of antigen. Although a small effect, this ability tended to reflect the activity of TF in the presence of antigen. It is concluded that neither the whole nor any significant part of this enhancement of transformation can be ascribed to an antigen-specific factor. Tuberculin-positive donors apparently yield a higher level of non-specific factor and possible reasons for this are discussed. The factor active in transformation may be responsbile for the TF phenomenon in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher M. S., Schneider W. J., Valentine F. T., Lawrence H. S. In vitro properties of leukocyte dialysates containing transfer factor. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1178–1182. doi: 10.1073/pnas.71.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R. Does transfer factor act specifically or as an immunologic adjuvant? N Engl J Med. 1973 Apr 26;288(17):908–909. doi: 10.1056/NEJM197304262881712. [DOI] [PubMed] [Google Scholar]
- Burger D. R., Vandenbark A. A., Finke P., Nolte J. E., Vetto R. M. Human transfer factor: effects on lymphocyte transformation. J Immunol. 1976 Sep;117(3):782–788. [PubMed] [Google Scholar]
- Casavant C. H., Youmans G. P. The adjuvant activity of mycobacterial RNA preparations and synthetic polynucleotides for induction of delayed hypersensitivity to purified protein derivative in guinea pigs. J Immunol. 1975 Mar;114(3):1014–1022. [PubMed] [Google Scholar]
- Chess L., Levy C., Schmukler M., Smith K., Mardiney M. R., Jr The effect of synthetic polynucleotides on immunologically induced tritiated thymidine incorporation. Amplification of response. Transplantation. 1972 Dec;14(6):748–755. doi: 10.1097/00007890-197212000-00013. [DOI] [PubMed] [Google Scholar]
- Cohen L., Holzman R. S., Valentine F. T., Lawrence H. S. Requirement of precommitted cells as targets for the augmentation of lymphocyte proliferation by leukocyte dialysates. J Exp Med. 1976 Apr 1;143(4):791–804. doi: 10.1084/jem.143.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. E., Johnson A. G. Regulation of the immune system by synthetic polynucleotides. IV. Amplification of proliferation of thymus-influenced lymphocytes. Cell Immunol. 1972 Feb;3(2):283–293. doi: 10.1016/0008-8749(72)90167-0. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Revillard J. P., Betuel H., Herzog C., Touraine J. L. Transfer factor therapy in immuno-deficiencies. Biomedicine. 1973 May;18(3):220–227. [PubMed] [Google Scholar]
- Littman B. H., Hirschman E. M., David J. R. Augmentation of 3H-thymidine incorporation by human lymphocytes in the presence of antigen and fractions of dialyzable transfer factor: a nonspecific phenomenon. Cell Immunol. 1977 Jan;28(1):158–166. doi: 10.1016/s0008-8749(77)80015-4. [DOI] [PubMed] [Google Scholar]
- Salaman M. R. Studies on the transfer factor of delayed hypersensitivity. Effect of dialysable leucocyte extracts from people of known tuberculin sensitivity on the migration of normal guinea-pig macrophages in the presence of antigen. Immunology. 1974 Jun;26(6):1069–1080. [PMC free article] [PubMed] [Google Scholar]
- Salaman M. R., Valdimarsson H. Letter: Specificity of transfer factor. Nature. 1976 Jan 22;259(5540):250–250. doi: 10.1038/259250b0. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Hambleton G., Henry K., McConnell I. Restoration of T-lymphocyte deficiency with dialysable leucocyte extract. Clin Exp Immunol. 1974 Feb;16(2):141–152. [PMC free article] [PubMed] [Google Scholar]
- Wilson G. B., Welch T. M., Fudenberg H. H. Tx: a component in human dialyzable transfer factor that induces cutaneous delayed hypersensitivity in guinea pigs. Clin Immunol Immunopathol. 1977 Mar;7(2):187–202. doi: 10.1016/0090-1229(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Wybran J., Levin A. S., Spitler L. E., Fudenberg H. H. Rosette-forming cells, immunologic deficiency diseases and transfer factor. N Engl J Med. 1973 Apr 5;288(14):710–713. doi: 10.1056/NEJM197304052881405. [DOI] [PubMed] [Google Scholar]


