Abstract
The course of the increased number of liver macrophages and the origin of these cells were studied after intravenous stimulation by zymosan, stilboestrol, or corynebacterium. The macrophages were isolated by digestion of the liver with pronase and DNAase.
Zymosan doubled the number of liver macrophages per gram of liver and led to a four- to five-fold increase in the number of blood monocytes, whereas stilboestrol induced a four-fold increase of liver macrophages and a two-fold increase of blood monocytes. Corynebacterium administration gave a two-fold rise in the number of liver macrophages and a six-fold increase of blood monocytes.
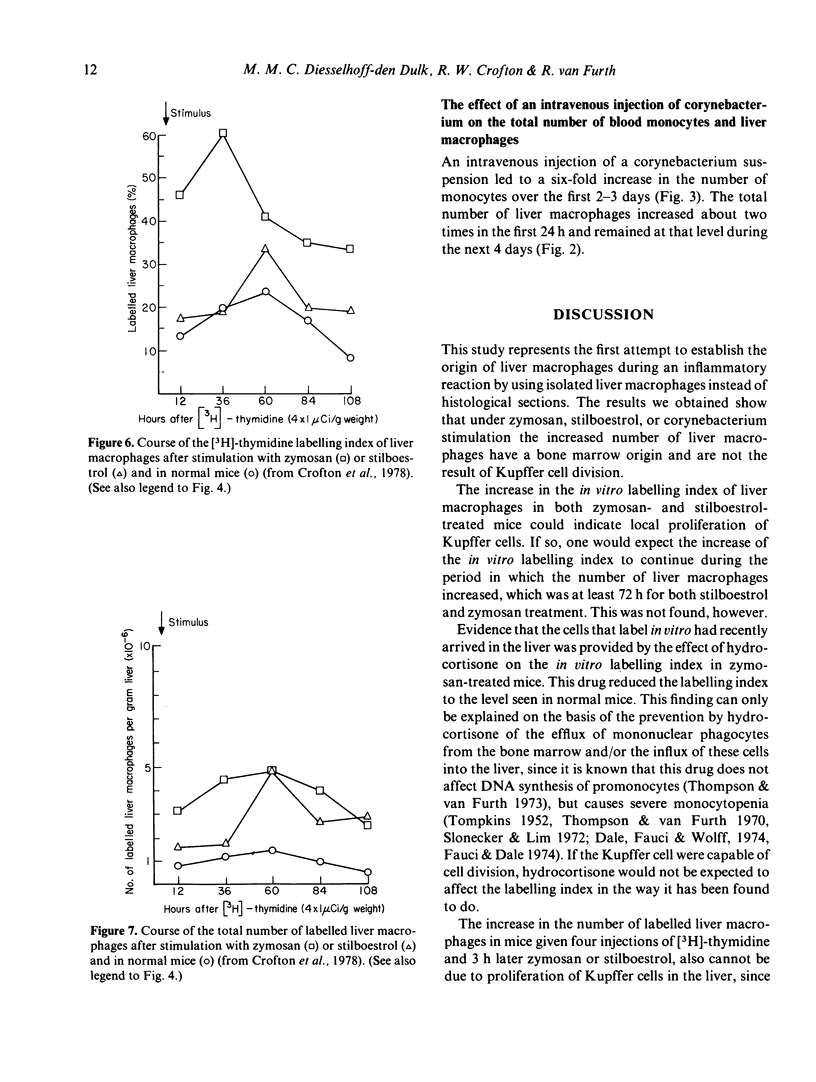
The in vitro labelling index of the liver macrophages showed a transient but marked increase after the administration of zymosan or stilboestrol, but returned to approximately normal values 4 days after the stimulus. Hydrocortisone given 48 h before zymosan prevented this increase in the in vitro labelling index of liver macrophages, thus demonstrating that the mononuclear phagocytes labelled in vitro had recently been recruited from the bone marrow to the liver.
Stimulation by stilboestrol or zymosan of mice labelled with [3H]-thymidine caused an increase in the number of labelled liver macrophages and blood monocytes as compared with the numerical course of the labelled Kupffer cells and monocytes in untreated mice. It may be concluded that this increase is attributable to the increased influx into the circulation of labelled monocytes from the bone marrow, which in turn migrate to the liver in larger numbers than are seen in the normal steady state. Local proliferation could not have been responsible for the increased number of labelled liver macrophages, because free [3H]-thymidine was no longer available when the stimulus was applied. Evidence supporting the bone marrow origin of the increased number of monocytes and liver macrophages after intravenous stimulation was provided by the course of the number of monocytes and liver macrophages in hydrocortisone-treated mice given zymosan as stimulus.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell E. B., Shand F. L. A search for lymphocyte-derived macrophages during xenogeneic graft-verus-host reactions induced by rat thoracic duct cells. Immunology. 1972 Apr;22(4):537–547. [PMC free article] [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D. C., Fauci A. S., Wolff S. M. Alternate-day prednisone. Leukocyte kinetics and susceptibility to infections. N Engl J Med. 1974 Nov 28;291(22):1154–1158. doi: 10.1056/NEJM197411282912203. [DOI] [PubMed] [Google Scholar]
- Emeis J. J., Planqué B. Heterogeneity of cells isolated from rat liver by pronase digestion: ultrastructure, cytochemistry and cell culture. J Reticuloendothel Soc. 1976 Jul;20(1):11–29. [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY L. S., BROWN B. A., DOBSON E. L. Cell division and phagocytic activity in liver reticulo-endothelial cells. Proc Soc Exp Biol Med. 1962 Jul;110:555–559. doi: 10.3181/00379727-110-27578. [DOI] [PubMed] [Google Scholar]
- Kelly L. S., Dobson E. L. Evidence concerning the origin of liver macrophages. Br J Exp Pathol. 1971 Feb;52(1):88–99. [PMC free article] [PubMed] [Google Scholar]
- Kinsky R. G., Christie G. H., Elson J., Howard J. G. Extra-hepatic derivation of Kupffer cells during oestrogenic stimulation of parabiosed mice. Br J Exp Pathol. 1969 Oct;50(5):438–447. [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand F. L., Bell E. B. Studies on the distribution of macrophages derived from rat bone marrow cells in xenogeneic radiation chimaeras. Immunology. 1972 Apr;22(4):549–556. [PMC free article] [PubMed] [Google Scholar]
- Slonecker C. E., Lim W. C. Effects of hydrocortisone on the cells in an acute inflammatory exudate. Lab Invest. 1972 Jul;27(1):123–128. [PubMed] [Google Scholar]
- Souhami R. L., Bradfield J. W. The recovery of hepatic phagocytosis after blockade of Kupffer cells. J Reticuloendothel Soc. 1974 Aug;16(2):75–86. [PubMed] [Google Scholar]
- TOMPKINS E. H. The response of monocytes to adrenal cortical extract. J Lab Clin Med. 1952 Mar;39(3):365–371. [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the kinetics of mononuclear phagocytes. J Exp Med. 1970 Mar 1;131(3):429–442. doi: 10.1084/jem.131.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the proliferation and kinetics of promonocytes and monocytes of the bone marrow. J Exp Med. 1973 Jan 1;137(1):10–21. doi: 10.1084/jem.137.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973 Dec 1;138(6):1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]
- Warr G. W., Sljivić V. S. Origin and division of liver macrophages during stimulation of the mononuclear phagocyte system. Cell Tissue Kinet. 1974 Nov;7(6):559–565. doi: 10.1111/j.1365-2184.1974.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Fahimi H. D. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. A light and electron microscopic cytochemical study. Am J Pathol. 1975 Sep;80(3):349–366. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]


