Abstract
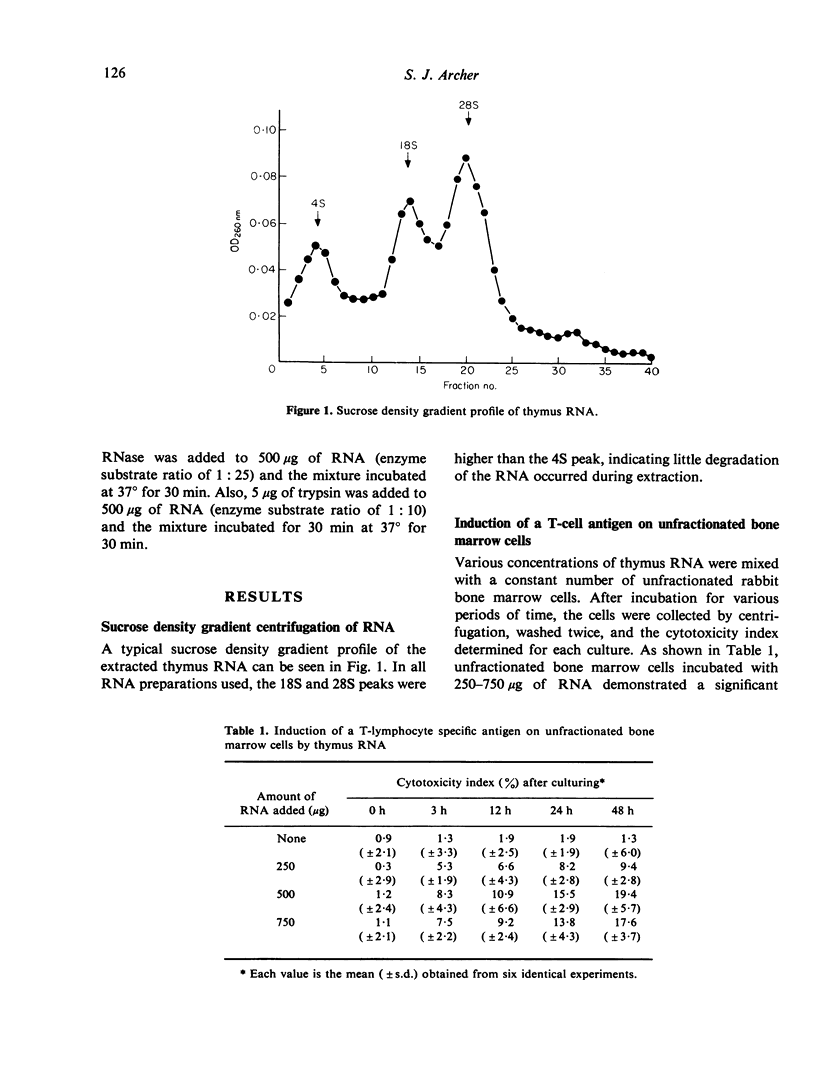
Expression of a rabbit T-cell specific antigen can be induced on bone marrow lymphocytes following exposure to an RNA extract obtained from the thymuses of young rabbits. The presence of the antigen was demonstrated using goat anti-rabbit T-cell serum in a complement-dependent cytotoxicity assay. The T-cell antigen first appeared 3 h after addition of the thymus RNA to bone marrow cell cultures and the maximum number of cells expressing the T-cell antigen was observed within 24 h. RNA obtained from a source other than the thymus was found to be ineffective in inducing expression of the T-cell antigen. The induction of the antigen appears to be dependent on the presence of intact thymus RNA, as RNase treatment but not trypsin treatment, destroyed the ability of the RNA to induce the T-cell antigen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch R. S., Goldstein G. Induction of T-cell differentiation in vitro by thymin, a purified polypeptide hormone of the thymus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1474–1478. doi: 10.1073/pnas.71.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch R. S., Goldstein G. Thymopoietin-induced acquisition of responsiveness to T cell mitogens. Cell Immunol. 1975 Dec;20(2):218–228. doi: 10.1016/0008-8749(75)90099-4. [DOI] [PubMed] [Google Scholar]
- Cohen E. P., Majer J., Friedman K. Responses to immunization in the thymus of the adult mouse. J Exp Med. 1969 Nov 1;130(5):1161–1174. doi: 10.1084/jem.130.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Hopper J. A., Goldstein A. L. Thymosin-induced differentiation of murine thymocytes in allogeneic mixed lymphocyte cultures. Ann N Y Acad Sci. 1975 Feb 28;249:145–153. doi: 10.1111/j.1749-6632.1975.tb29064.x. [DOI] [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradelizi D. P., Chou C. T., Cinader B., Dubiski S. A membrane antigen of rabbit thymus cells. Cell Immunol. 1973 Jun;7(3):484–501. doi: 10.1016/0008-8749(73)90212-8. [DOI] [PubMed] [Google Scholar]
- Friedman H. Introcutory remarks to the conference on "Thymus Factors in Immunity". Ann N Y Acad Sci. 1975 Feb 28;249:5–8. doi: 10.1111/j.1749-6632.1975.tb29051.x. [DOI] [PubMed] [Google Scholar]
- Friedman H. RNA in the immune response. Introductory remarks. Ann N Y Acad Sci. 1973 May 31;207:5–7. doi: 10.1111/j.1749-6632.1973.tb47471.x. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Asanuma Y., Battisto J. R., Hardy M. A., Quint J., White A. Influence of thymosin on cell-mediated and humoral immune responses in normal and in immunologically deficient mice. J Immunol. 1970 Feb;104(2):359–366. [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Low T. L., McAdoo M., McClure J., Thurman G. B., Rossio J., Lai C. Y., Chang D., Wang S. S., Harvey C. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. Proc Natl Acad Sci U S A. 1977 Feb;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. The isolation of thymopoietin (thymin). Ann N Y Acad Sci. 1975 Feb 28;249:177–185. doi: 10.1111/j.1749-6632.1975.tb29067.x. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Boumsell L., Touraine J. L., Espérance P. L., Smithwick E., O'Reilly R., Good R. A. Enhancement of T-lymphocyte differentiation in vitro by thymic extracts after bone marrow transplantation in severe combined immunodeficiencies. Clin Immunol Immunopathol. 1975 Jul;4(2):258–268. doi: 10.1016/0090-1229(75)90061-6. [DOI] [PubMed] [Google Scholar]
- Isaković K., Mitrović K., Marković B. M., Rajcević M., Janković B. D. Preparation of specific anti-thymocyte and anti-bursacyte sera in rabbits. J Immunol Methods. 1975 Jul;7(4):359–369. doi: 10.1016/0022-1759(75)90044-7. [DOI] [PubMed] [Google Scholar]
- Nakumura K. The proliferation of plasma cells from mouse bone marrow in vitro. II-Stimulation of IgG-producing cells by a RNase-sensitive thymocyte homogenate. Cell Immunol. 1976 Aug;25(2):163–177. doi: 10.1016/0008-8749(76)90107-6. [DOI] [PubMed] [Google Scholar]
- Rotter V., Schlesinger M., Kalderon R., Trainin N. Response of human lymphocytes to PHA and Con A, dependent on and regulated by THF, a thymic hormone. J Immunol. 1976 Nov;117(5 PT2):1927–1932. [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]


