Abstract
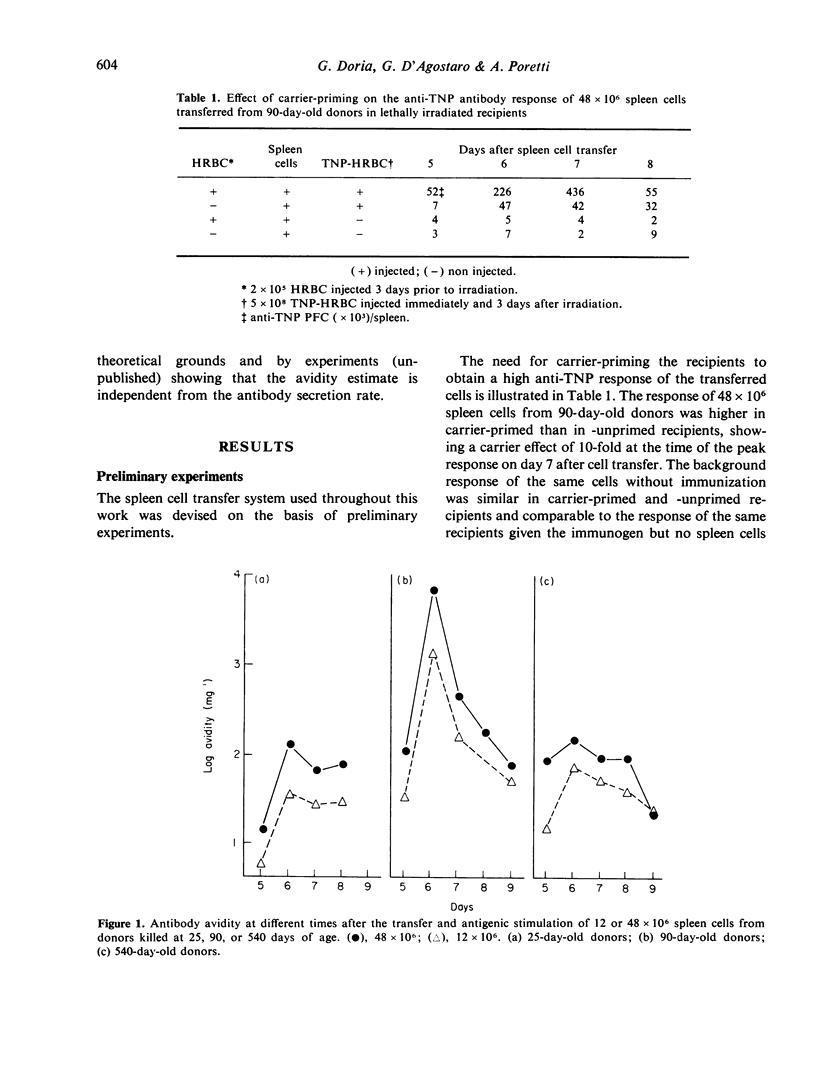
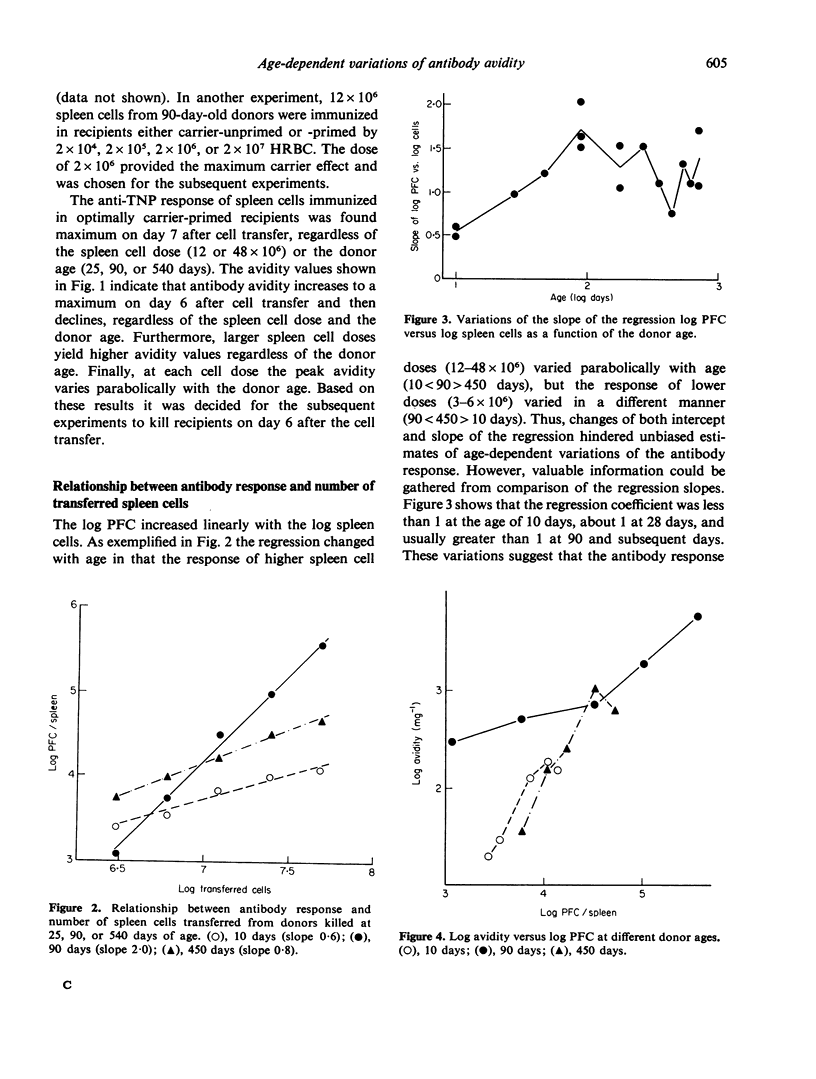
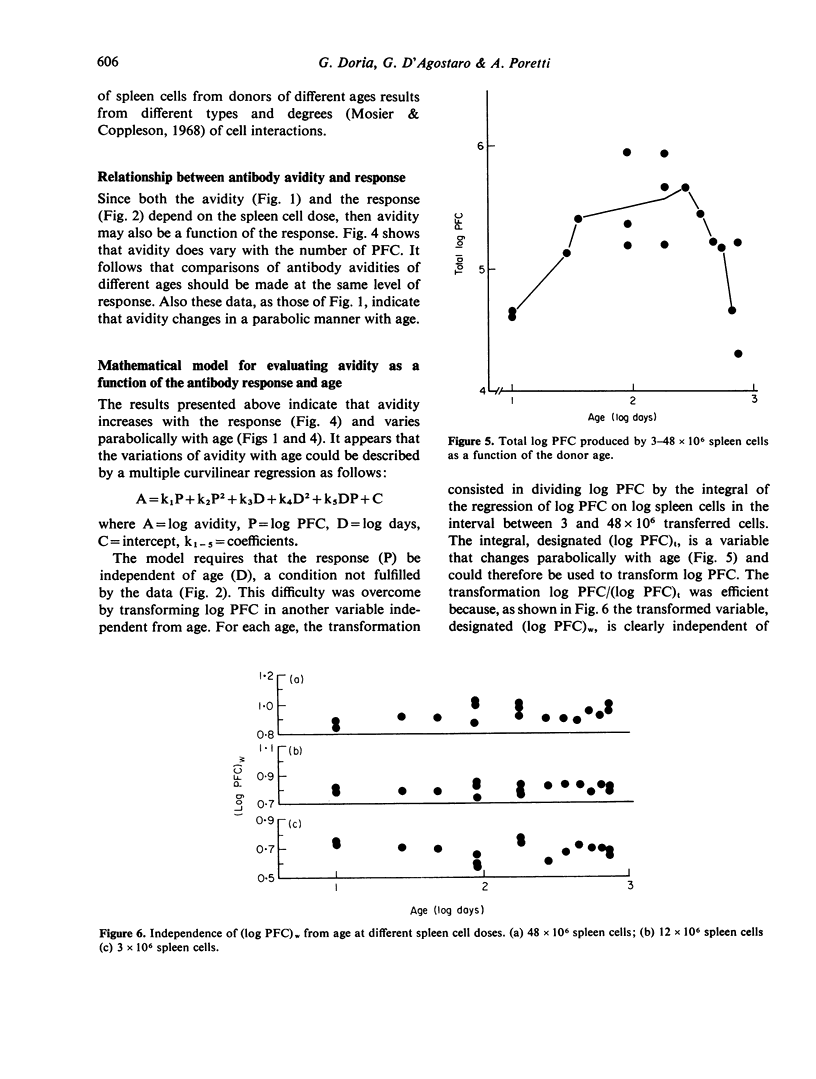
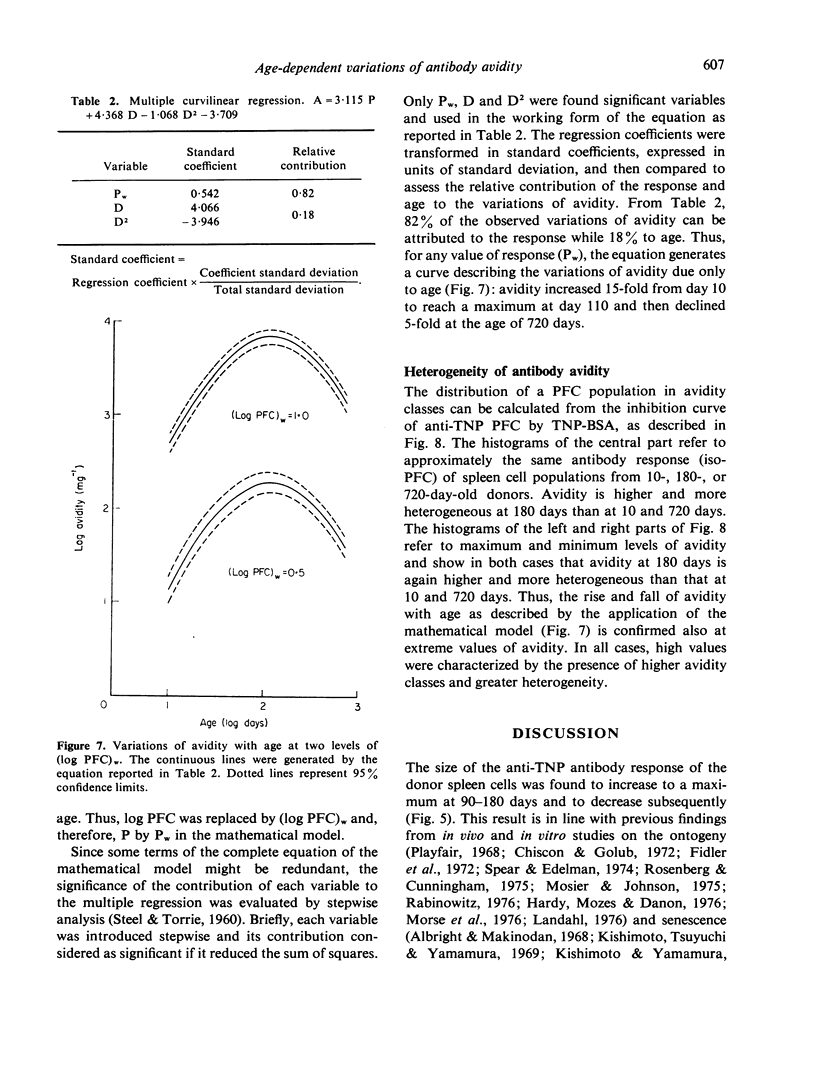
Age-dependent variations of antibody avidity were studied in the C3HeB/FeJ mouse. Spleen cells from donors of different ages (10--720 days) were transferred and stimulated with TNP-HRBC in lethally irradiated syngenic recipients. The anti-TNP antibody response of the donor cells was estimated from the number of direct PFC per recipient spleen by the Jerne technique with TNP-SRBC. Avidity of the antibodies secreted by PFC was evaluated from the amount of added TNP-BSA that inhibited 50% of the anti-TNP PFC. Under these experimental conditions allowing the exclusion of any influence of the donor milieu during the immune response, age-dependent variations of the antibody response and avidity could be attributed to changes in the donor spleen cell population. Avidity was found to increase with the response and to vary parabolically with age. After appropriate correction of the number of PFC to make it independent from age, avidity values were fitted by a multiple curvilinear regression in which the independent variables playing a significant role were the corrected number of PFC in its linear term and the age in its linear and quadratic terms. From comparison of the standard coefficients of this regression, the observed variations of avidity could be attributed in part (82%) to the response and in part (18%) to the age. For any value of response, avidity increased 15-fold from day 10 to reach a maximum at day 110 and then declined 5-fold at the age of 720 days. Heterogeneity of avidity also changed parabolically with age as high avidity classes were present in adulthood and absent at 10 and 720 days.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. F., Makinodan T. Growth and senescence of antibody-forming cells. J Cell Physiol. 1966 Jun;67(3 Suppl):185+–185+. doi: 10.1002/jcp.1040670415. [DOI] [PubMed] [Google Scholar]
- Albright J. W., Makinodan T. Decline in the growth potential of spleen-colonizing bone marrow stem cells of long-lived aging mice. J Exp Med. 1976 Nov 2;144(5):1204–1213. doi: 10.1084/jem.144.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. II. Linear transmission of cellular memory. J Exp Med. 1967 Feb 1;125(2):199–211. doi: 10.1084/jem.125.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F., Schmidt D., Strom R. Determination of avidity of anti-albumin antibodies in the mouse. Influence of the number of cells transferred on the quality of the secondary adoptive response. Immunology. 1969 Aug;17(2):189–198. [PMC free article] [PubMed] [Google Scholar]
- Chen M. G. Impaired Elkind recovery in hematopoietic colony-forming cells of aged mice. Proc Soc Exp Biol Med. 1974 Apr;145(4):1181–1186. doi: 10.3181/00379727-145-37977. [DOI] [PubMed] [Google Scholar]
- Chiscon M. Q., Golub E. S. Functional development of the interacting cells in the immune response. I. Development of T cell and B cell function. J Immunol. 1972 May;108(5):1379–1386. [PubMed] [Google Scholar]
- Coppleson L. W., Michie D. A quantitative study of the chorioallantoic membrane reaction in the chick embryo. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):555–563. doi: 10.1098/rspb.1966.0009. [DOI] [PubMed] [Google Scholar]
- D'Eustachio P., Edelman G. M. Frequency and avidity of specific antigen-binding cells in developing mice. J Exp Med. 1975 Nov 1;142(5):1078–1091. doi: 10.1084/jem.142.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C. Hemolytic plaque inhibition: the physical chemical limits on its use as an affinity assay. J Immunol. 1976 Dec;117(6):2249–2257. [PubMed] [Google Scholar]
- Doria G., Agarossi G., Boraschi D., Antonietta M. Effect of carrier priming on antibody avidity in the in vivo and in vitro immune response. Immunology. 1977 Apr;32(4):539–548. [PMC free article] [PubMed] [Google Scholar]
- Falkoff R., Kettman J. Differential stimulation of precursor cells and carrier-specific thymus-derived cell activity in the in vivo reponse to heterologous erythrocytes in mice. J Immunol. 1972 Jan;108(1):54–58. [PubMed] [Google Scholar]
- Farrar J. J., Loughman B. E., Nordin A. A. Lymphopoietic potential of bone marrow cells from aged mice: comparison of the cellular constituents of bone marrow from young and aged mice. J Immunol. 1974 Mar;112(3):1244–1249. [PubMed] [Google Scholar]
- Fidler J. M., Chiscon M. O., Golub E. S. Functional development of the interacting cells in the immune response. II. Development of immunocompetence to heterologous erythrocytes in vitro. J Immunol. 1972 Jul;109(1):136–140. [PubMed] [Google Scholar]
- Gelfand M. C., Elfenbein G. J., Frank M. M., Paul W. E. Ontogeny of B lymphocytes. II. Relative rates of appearance of lymphocytes bearing surface immunoglobulin and complement receptors. J Exp Med. 1974 May 1;139(5):1125–1141. doi: 10.1084/jem.139.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbase-DeLima M., Wilkinson J., Smith G. S., Walford R. L. Age-related decline in thymic-independent immune function in a long-lived mouse strain. J Gerontol. 1974 May;29(3):261–268. doi: 10.1093/geronj/29.3.261. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Goidl E. A., Innes J. B., Weksler M. E. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976 Oct 1;144(4):1037–1048. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goidl E. A., Klass J., Siskind G. W. Ontogeny of B-lymphocyte function. II. Ability of endotoxin to increase the heterogeneity of affinity of the immune response of B lymphocytes from fetal mice. J Exp Med. 1976 Jun 1;143(6):1503–1520. doi: 10.1084/jem.143.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goidl E. A., Siskind G. W. Ontogeny of B-lymphocyte function. I. Restricted heterogeneity of the antibody response of B lymphocytes from neonatal and fetal mice. J Exp Med. 1974 Nov 1;140(5):1285–1302. doi: 10.1084/jem.140.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. J., Lajtha L. G. Kinetic study of the production of antibody-forming cells from their precursors. Nature. 1968 Jun 15;218(5146):1079–1081. doi: 10.1038/2181079a0. [DOI] [PubMed] [Google Scholar]
- Hardy B., Mozes E., Danon D. Comparison of the immune response potential of newborn mice to T-dependent and T-independent synthetic polypeptides. Immunology. 1976 Feb;30(2):261–266. [PMC free article] [PubMed] [Google Scholar]
- Heidrick M. L., Makinodan T. Presence of impairment of humoral immunity in nonadherent spleen cells of old mice. J Immunol. 1973 Nov;111(5):1502–1506. [PubMed] [Google Scholar]
- Hirokawa K., Makinodan T. Thymic involution: effect on T cell differentiation. J Immunol. 1975 Jun;114(6):1659–1664. [PubMed] [Google Scholar]
- Hori Y., Perkins E. H., Halsall M. K. Decline in phytohemagglutinin responsiveness of spleen cells from aging mice. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):48–53. doi: 10.3181/00379727-144-37524. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. An in vitro primary immune response to 2,4,6-trinitrophenyl substituted erythrocytes: response against carrier and hapten. J Immunol. 1970 Jun;104(6):1558–1561. [PubMed] [Google Scholar]
- Kishimoto S., Takahama T., Mizumachi H. In vitro immune response to the 2,4,6-trinitrophenyl determinant in aged C57BL/6J mice:changes in the humoral immune response to, avidity for the TNP determinant and responsiveness to LPS effect with aging. J Immunol. 1976 Feb;116(2):294–300. [PubMed] [Google Scholar]
- Kishimoto S., Tsuyuguchi I., Yamamura Y. Immune responses in aged mice. Clin Exp Immunol. 1969 Nov;5(5):525–530. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto S., Yamamura Y. Immune responses in aged mice: changes of antibody-forming cell precursors and antigen-reactive cells with ageing. Clin Exp Immunol. 1971 Jun;8(6):957–962. [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Press J. L. The characterization fo the B-cell repertoire specific for the 2,4-dinitrophenyl and 2,4,6-trinitrophenyl determinants in neonatal BALB/c mice. J Exp Med. 1975 May 1;141(5):1133–1146. doi: 10.1084/jem.141.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972 Aug 1;136(2):241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landahl C. A. Ontogeny of adherent cells. I. Distribution and ontogeny of A cells participating in the response to sheep erythrocytes in vitro. Eur J Immunol. 1976 Feb;6(2):130–134. doi: 10.1002/eji.1830060212. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Adler W. H. Effects of aging on the differentiation and proliferation potentials of cells of the immune system. Fed Proc. 1975 Feb;34(2):153–158. [PubMed] [Google Scholar]
- Makinodan T., Albright J. W., Good P. I., Peter C. P., Heidrick M. L. Reduced humoral immune activity in long-lived old mice: an approach to elucidating its mechanisms. Immunology. 1976 Dec;31(6):903–911. [PMC free article] [PubMed] [Google Scholar]
- Marshall-Clarke S., Playfair J. H. Age-dependent changes in the relative affinity of anti-dinitrophenyl antibodies in mice. Immunology. 1975 Sep;29(3):477–486. [PMC free article] [PubMed] [Google Scholar]
- Medvedev Z. A. Repetition of molecular-genetic information as a possible factor in evolutionary changes of life span. Exp Gerontol. 1972 Aug;7(4):227–238. doi: 10.1016/0531-5565(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Meredith P., Gerbase-DeLima M., Walford R. L. Age-related changes in the PHA: con A stimulatory ratios of cells from spleens of a long-lived mouse strain. Exp Gerontol. 1975;10(5):247–250. doi: 10.1016/0531-5565(75)90002-9. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Prescott B., Cross S. S., Stashak P. W., Baker P. J. Regulation of the antibody response to type III pneumococcal polysaccharide. V. Ontogeny of factors influencing the magnitude of the plaque-forming cell response. J Immunol. 1976 Feb;116(2):279–287. [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M. Ontogeny of mouse lymphocyte function. II. Development of the ability to produce antibody is modulated by T lymphocytes. J Exp Med. 1975 Jan 1;141(1):216–226. doi: 10.1084/jem.141.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgita R. A., Tomasi T. B., Jr Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975 Feb 1;141(2):269–286. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North J. R., Feinstein A. Analysis of anti-hapten foci in repopulated mouse spleens. Eur J Immunol. 1973 Dec;3(12):784–789. doi: 10.1002/eji.1830031209. [DOI] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press J. L., Klinman N. R. Enumeration and analysis of antibody-forming cell precursors in the neonatal mouse. J Immunol. 1973 Sep;111(3):829–835. [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. I. Characterization of intrinsic deficiencies. J Immunol. 1972 Feb;108(2):403–412. [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. II. Characterization of extrinsic deficiencies. J Immunol. 1972 Feb;108(2):413–417. [PubMed] [Google Scholar]
- Rabinowitz S. G. Measurement and comparison of the proliferative and antibody response of neonatal, immature and adult murine spleen cells to T-dependent and T-independent antigens. Cell Immunol. 1976 Feb;21(2):201–216. doi: 10.1016/0008-8749(76)90049-6. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y. J., Cunningham A. J. Ontogeny of the antibody-forming cell line in mice. I. Kinetics of appearance of mature B cells. Eur J Immunol. 1976 Jul;5(7):444–447. doi: 10.1002/eji.1830050703. [DOI] [PubMed] [Google Scholar]
- Scher I., Sharrow S. O., Wistar R., Jr, Asofsky R., Paul W. E. B-lymphocyte heterogeneity: ontogenetic development and organ distribution of B-lymphocyte populations defined by their density of surface immunoglobulin. J Exp Med. 1976 Aug 1;144(2):494–506. doi: 10.1084/jem.144.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre D., Segre M. Humoral immunity in aged mice. II. Increased suppressor T cell activity in immunologically deficient old mice. J Immunol. 1976 Mar;116(3):735–738. [PubMed] [Google Scholar]
- Segre M., Segre D. Humoral immunity in aged mice. I. Age-related decline in the secondary response to DNP of spleen cells propagated in diffusion chambers. J Immunol. 1976 Mar;116(3):731–734. [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Development of B lymphocytes. I. Cell populations and a critical event during ontogeny. J Immunol. 1975 Jun;114(6):1730–1735. [PubMed] [Google Scholar]
- Spear P. G., Edelman G. M. Maturation of the humoral immune response in mice. J Exp Med. 1974 Feb 1;139(2):249–263. doi: 10.1084/jem.139.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler B., Hirsch G., Gusseck D., Johnson R., Bick M. Codon-restriction theory by aging and development. J Theor Biol. 1971 Dec;33(3):429–474. doi: 10.1016/0022-5193(71)90091-9. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Takemori T. Properties of primed suppressor T cells and their products. Transplant Rev. 1975;26:106–129. doi: 10.1111/j.1600-065x.1975.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Takemori T., Tada T. Selective roles of thymus-derived lymphocytes in the antibody response. II. Preferential suppression of high-affinity antibody-forming cells by carrier-primed suppressor T cells. J Exp Med. 1974 Jul 1;140(1):253–266. doi: 10.1084/jem.140.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Melcher U., McWilliams M., Lamm M. E., Phillips-Quagliata J. M., Uhr J. W. Cell surface immunoglobulin. XI. The appearance of an IgD-like molecule on murine lymphoid cells during ontogeny. J Exp Med. 1975 Jan 1;141(1):206–215. doi: 10.1084/jem.141.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hahn H. P. Failures of regulation mechanisms as causes of cellular aging. Adv Gerontol Res. 1971;3:1–38. [PubMed] [Google Scholar]


