Abstract
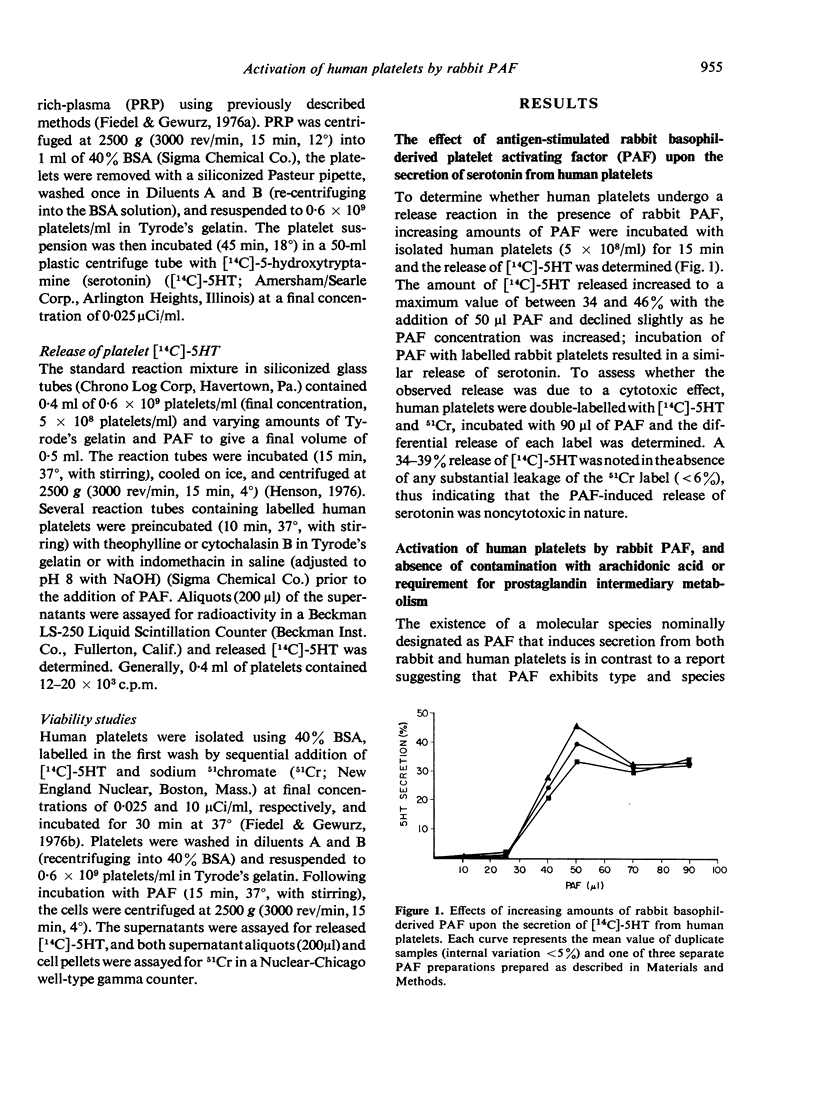
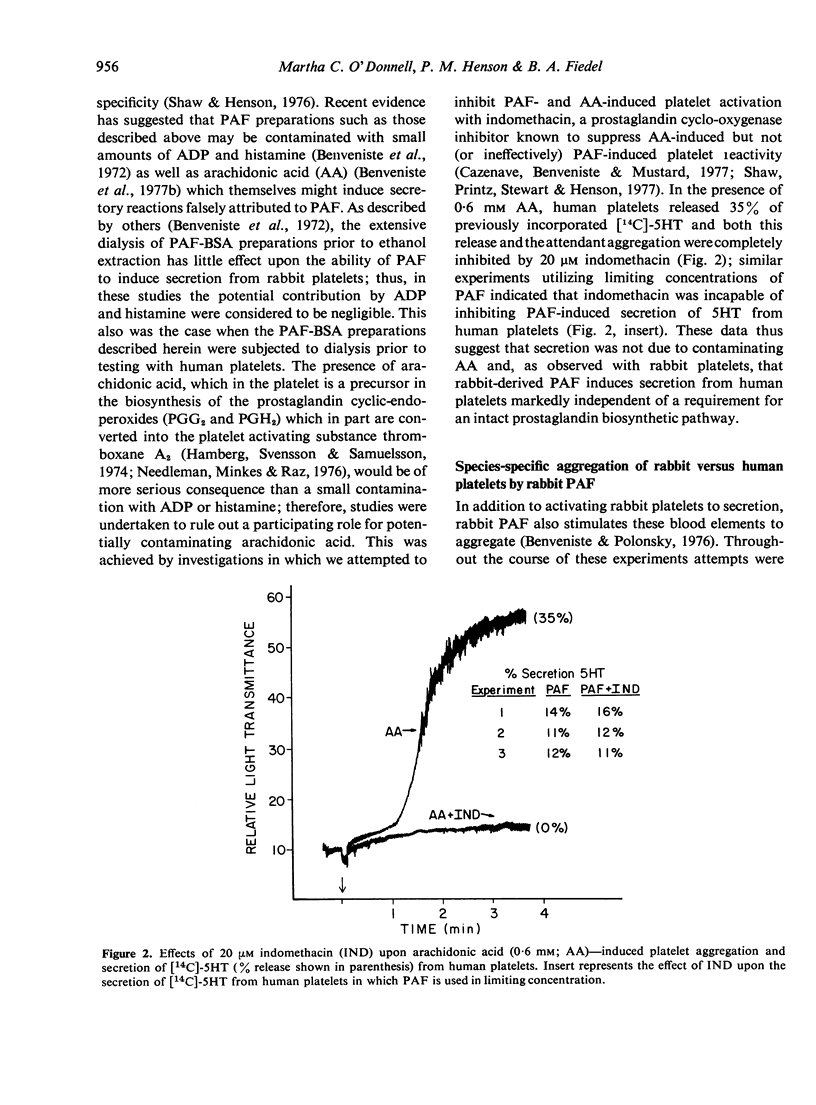
Rabbit basophil-derived platelet activating factor (PAF), a mediator of anaphylaxis, induces the aggregation and release of serotonin from rabbit platelets. In the present study, we report that PAF obtained by challenge of specifically sensitized rabbit basophils induced the noncytotoxic release of serotonin from human platelets; maximal extent of release ranged between 34-46%. This release was unaltered in the presence of indomethacin, indicating that such secretion was not a consequence of contaminating arachidonic acid; further, as previously demonstrated with platelets of rabbit origin, it was markedly independent of a requirement for an intact prostaglandin biosynthetic pathway. In contrast to its effect upon rabbit platelets, rabbit PAF did not induce aggregation of human platelets, suggesting that the aggregation and secretion reactions induced by this agent are separable and that this cross-species activation may be incomplete. Whether this is a result of the differential ability of rabbit PAF to bind to and activate rabbit as compared to human platelets or to the existence of a family of PAF molecules is not yet known. The capacity of PAF to participate in a secretory event involving human platelets lends support to the belief that PAF may play an important ubiquitous role in the cooperative, leucocyte-dependent, release of vasoactive amines which results in increased vascular permeability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbaro J. F., Zvaifler N. J. Antigen induced histamine release from platelets of rabbits producing homologous PCA antibody. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1245–1247. doi: 10.3181/00379727-122-31371. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Le Couedic J. P., Polonsky J., Tence M. Structural analysis of purified platelet-activating factor by lipases. Nature. 1977 Sep 8;269(5624):170–171. doi: 10.1038/269170a0. [DOI] [PubMed] [Google Scholar]
- Benveniste J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature. 1974 Jun 7;249(457):581–582. doi: 10.1038/249581a0. [DOI] [PubMed] [Google Scholar]
- Fiedel B. A., Gewurz H. Effects of C-reactive protein on platelet function. I. Inhibition of platelet aggregation and release reactions. J Immunol. 1976 May;116(5):1289–1294. [PubMed] [Google Scholar]
- Fiedel B. A., Gewurz H. Effects of C-reactive protein on platelet function. II. Inhibition by CRP of platelet reactivities stimulated by poly-L-lysine, ADP, epinephrine, and collagen. J Immunol. 1976 Oct;117(4):1073–1078. [PubMed] [Google Scholar]
- Fiedel B. A., Simpson R. M., Gewurz H. Effects of C-reactive protein on platelet function. III. The role of cAMP, contractile elements, and prostaglandin metabolism in CRP-induced inhibition of platelet aggregation and secretion. J Immunol. 1977 Sep;119(3):877–882. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Activation and desensitization of platelets by platelet-activating factor (PAF) derived from IgE-sensitized basophils. I. Characteristics of the secretory response. J Exp Med. 1976 Apr 1;143(4):937–952. doi: 10.1084/jem.143.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Activation of platelets by platelet-activating factor (PAF) derived from IgE-sensitized basophils. II. The role of serine proteases, cyclic nucleotides, and contractile elements in PAF-induced secretion. J Exp Med. 1976 Apr 1;143(4):953–968. doi: 10.1084/jem.143.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. doi: 10.1084/jem.131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravis T. C., Henson P. M. IgE-induced release of a platelet-activating factor from rabbit lung. J Immunol. 1975 Dec;115(6):1677–1681. [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]


