Abstract
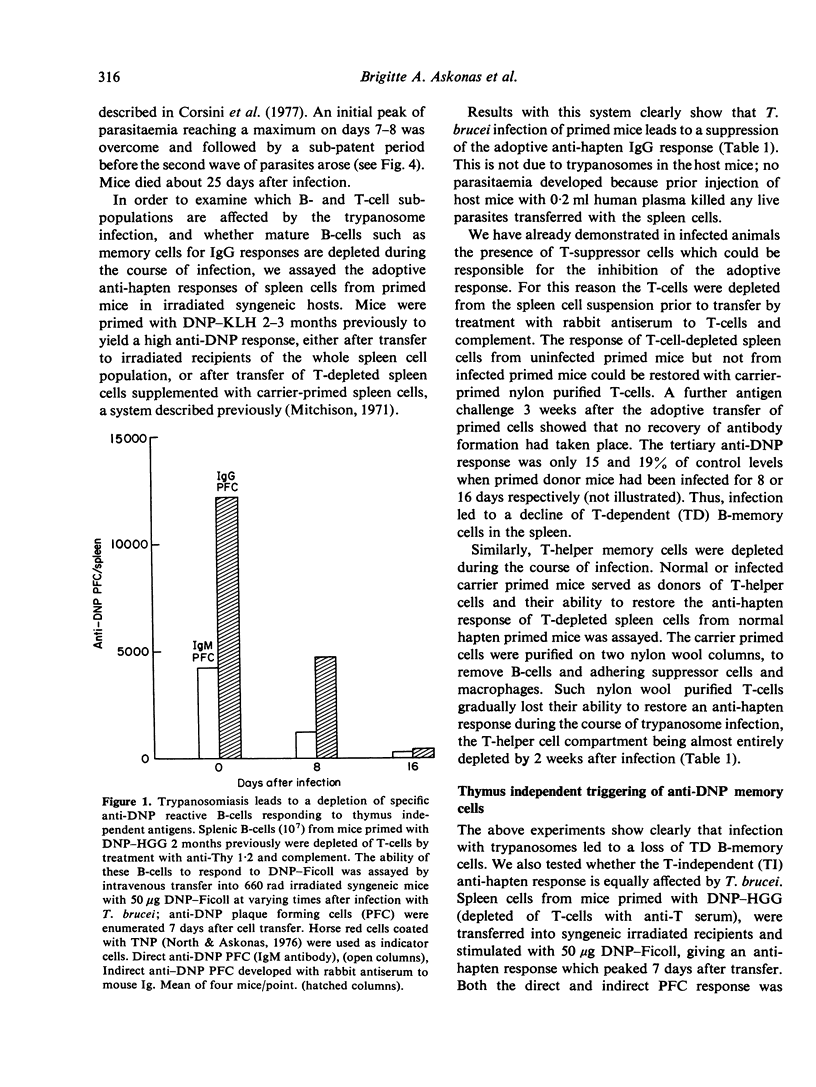
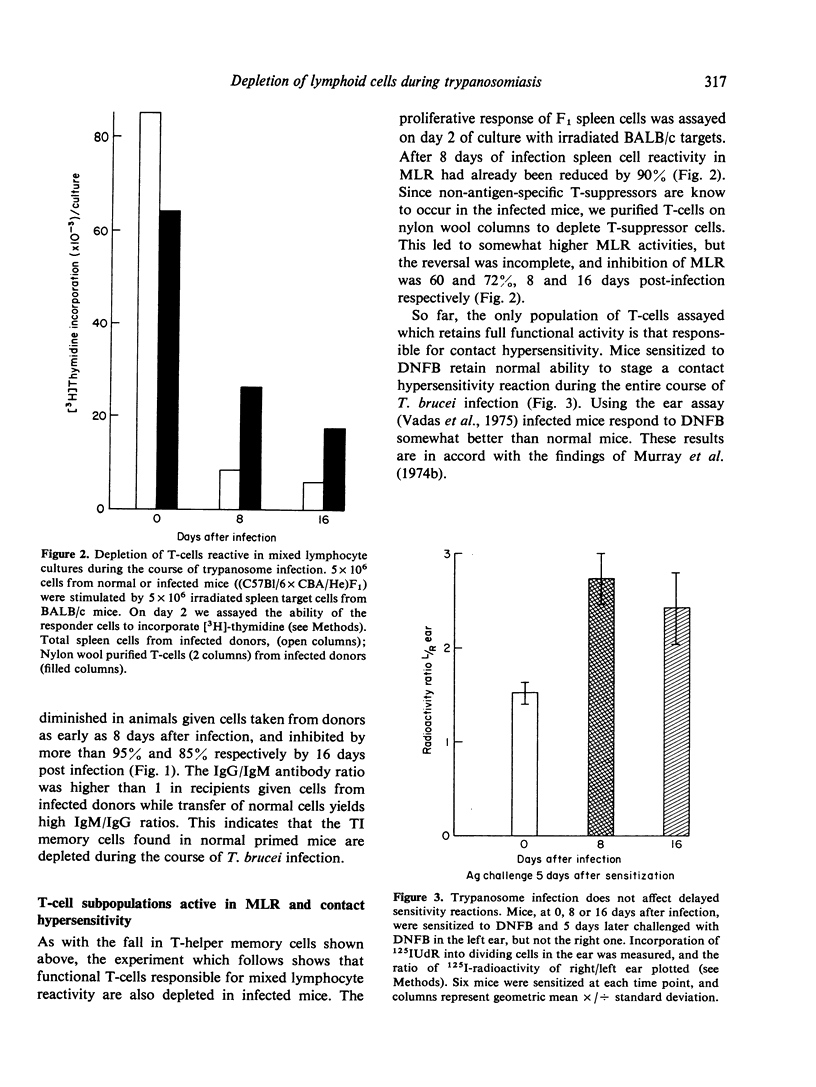
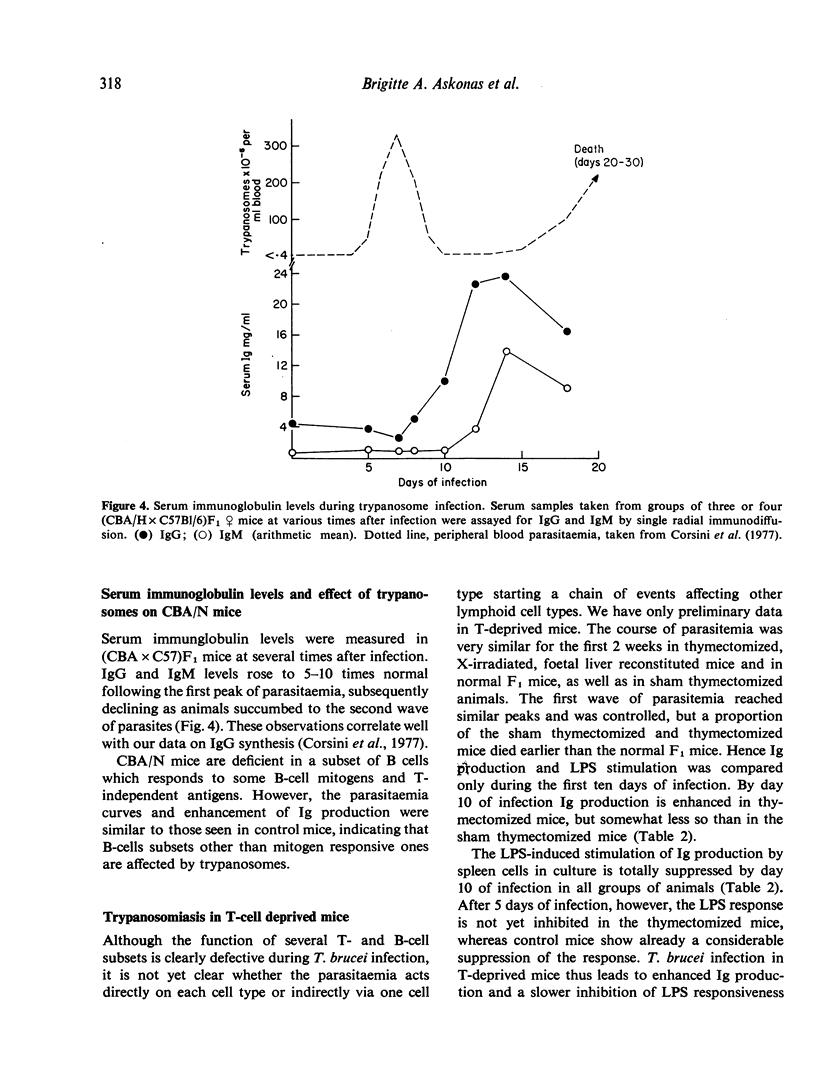
T. brucei infection in mice causes generalized immunosuppression with multiple changes in the cells of the lymphoid tissue. Loss of B cell responsiveness to antigens and mitogens, and the induction of suppressive T-cells and macrophages, have been previously reported (Hudson, Byner, Freeman & Terry, 1976; Corsini, Clayton, Askonas & Ogilvie, 1977; Jayawardena & Waksman, 1977). In this study, purified B- or T-cell populations from infected mice have been tested functionally in vitro or in vivo by transfer into syngeneic irradiated hosts to separate the cells from trypanosomes or their products. B-memory cells for thymus dependent (DNP-KLH) and thymus independent (DNP-Ficoll) antigens are depleted or lose their potential to respond to the antigen during T. brucei infection. Similarly, purified T-helper cells, and T-cells reactive to allogeneic target cells in mixed lymphocyte reactions are functionally defective. By 16 days of infection all these responses are less than 10% of the normal level. The loss of B-cell function follows the peak parasitaemia and is accompanied by increases in the serum levels of both IgM and IgG. Enhanced Ig production and decline in B-cell potential also occur in T-deprived mice and in CBA/N mice which lack a subset of T-independent B-cells. Cells affecting delayed hypersensitivity reactions retain their activity throughout trypanosome infection and so far provide the only exception to the general decline in immune potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Williamson A. R. Factors affecting the propagation of a B cell clone forming antibody to the 2,4-dinitrophenyl group. Eur J Immunol. 1972 Dec;2(6):487–493. doi: 10.1002/eji.1830020603. [DOI] [PubMed] [Google Scholar]
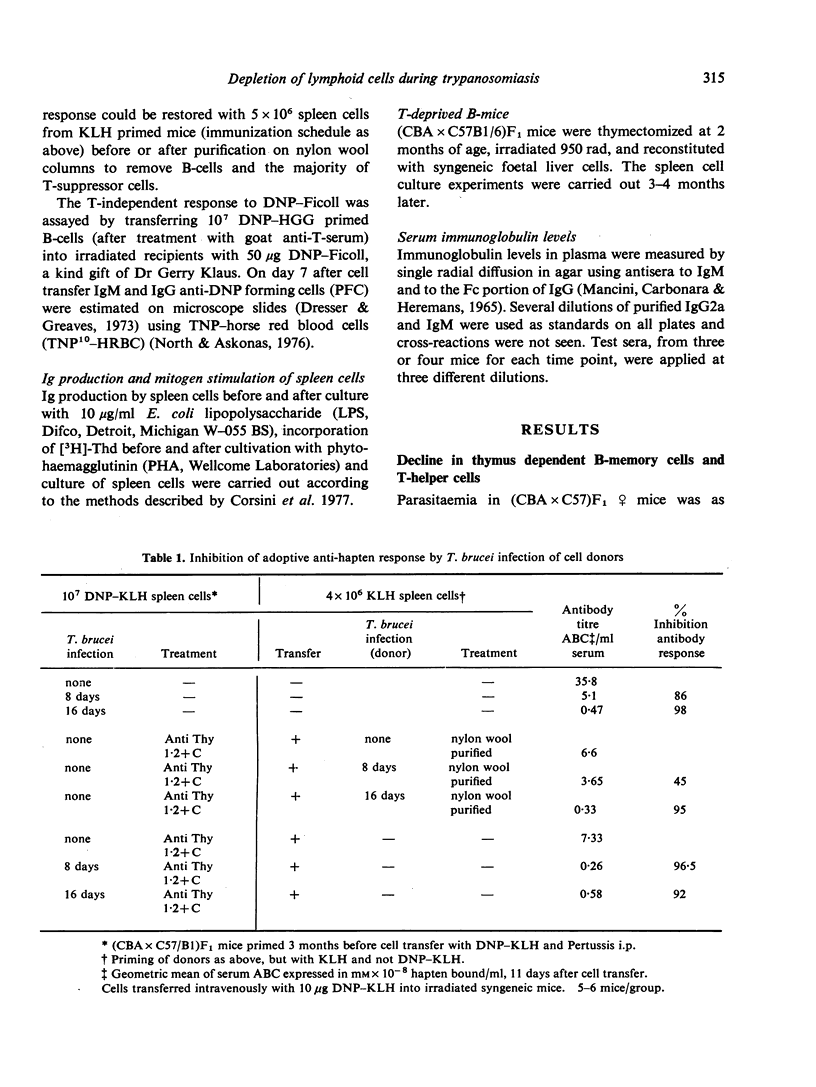
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Goodwin L. G., Green D. G., Guy M. W., Voller A. Immunosuppression during trypanosomiasis. Br J Exp Pathol. 1972 Feb;53(1):40–43. [PMC free article] [PubMed] [Google Scholar]
- Houba V., Brown K. N., Allison A. C. Heterophile antibodies, M-antiglobulins and immunoglobulins in experimental trypanosomiasis. Clin Exp Immunol. 1969 Jan;4(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Luckins A. G., Mehlitz D. Observations on serum immunoglobulin levels in cattle infected with Trypanosoma brucei, T. vivax and T. congolense. Ann Trop Med Parasitol. 1976 Dec;70(4):479–480. doi: 10.1080/00034983.1976.11687153. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- North J. R., Askonas B. A. IgG response in vitro. I. The requirement for an intermediate responsive cell type. Eur J Immunol. 1976 Jan;6(1):8–15. doi: 10.1002/eji.1830060104. [DOI] [PubMed] [Google Scholar]
- North J. R., Kemshead J. T., Askonas B. A. Non-specific factor replaces T cells in an IgG response to soluble antigens. Immunology. 1977 Sep;33(3):321–329. [PMC free article] [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., Gamble J., Whitelaw A. A radioisotopic method to measure delayed type hypersensitivity in the mouse. I. Studies in sensitized and normal mice. Int Arch Allergy Appl Immunol. 1975;49(5):670–692. doi: 10.1159/000231449. [DOI] [PubMed] [Google Scholar]


