Abstract
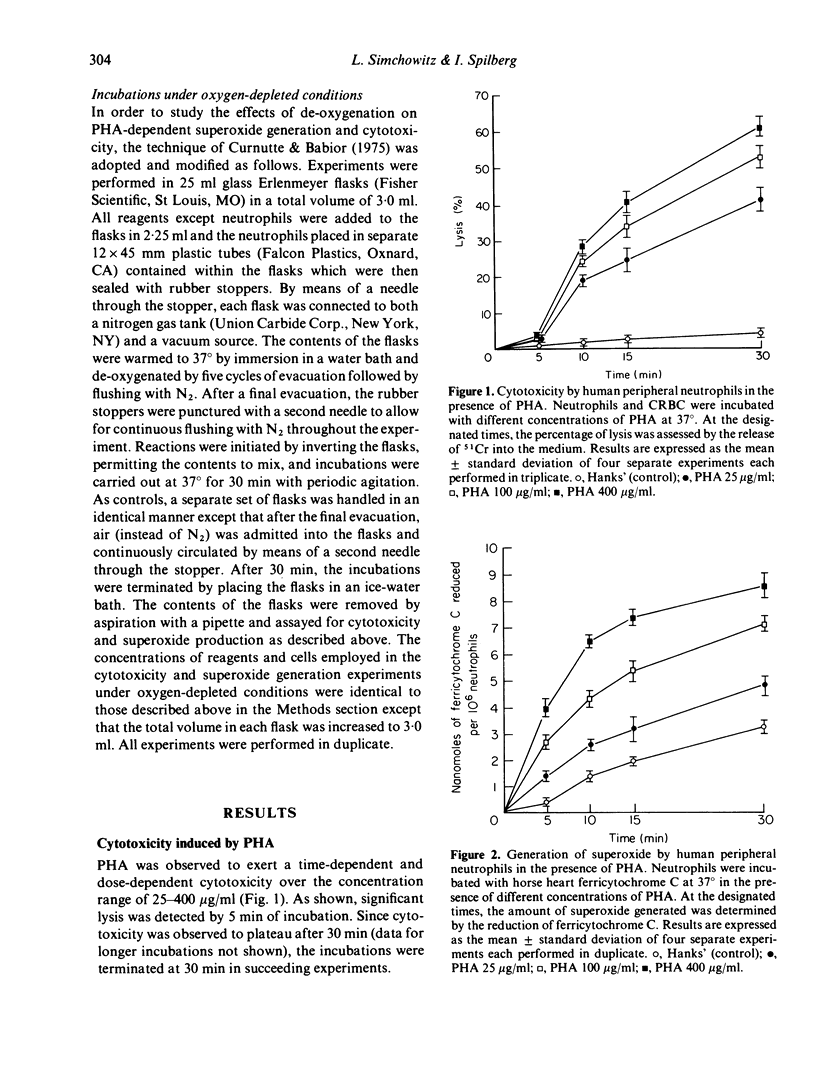
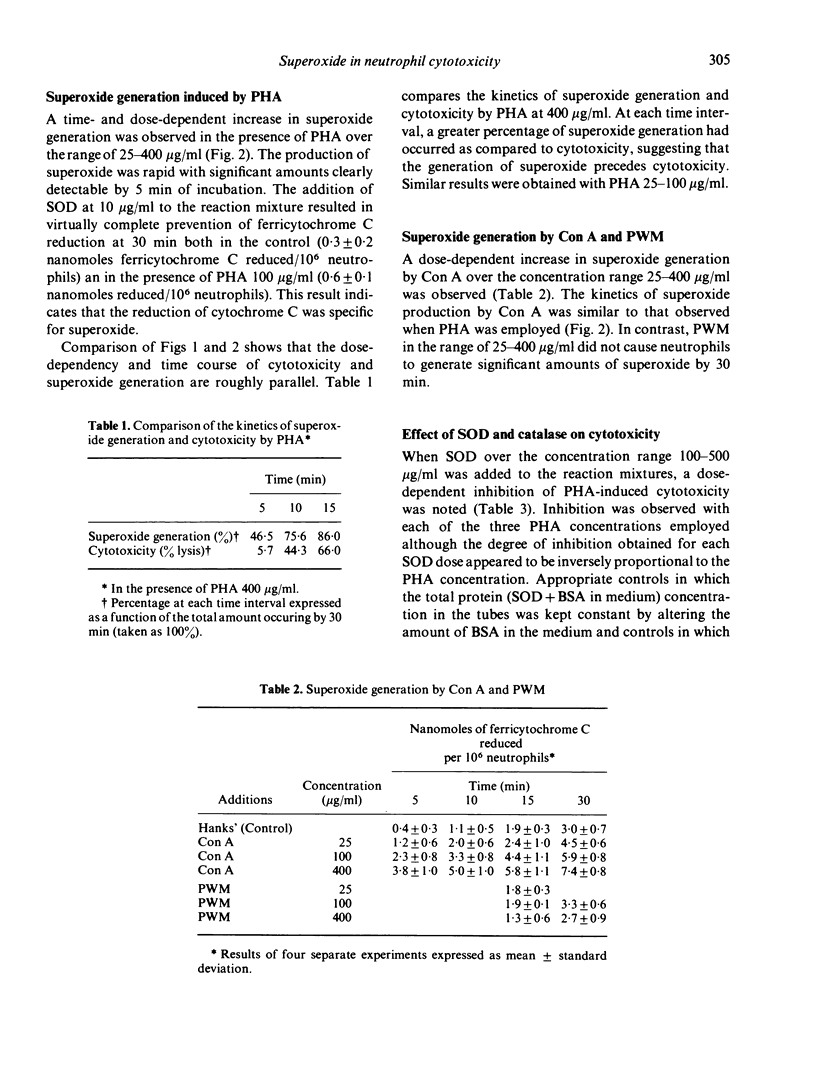
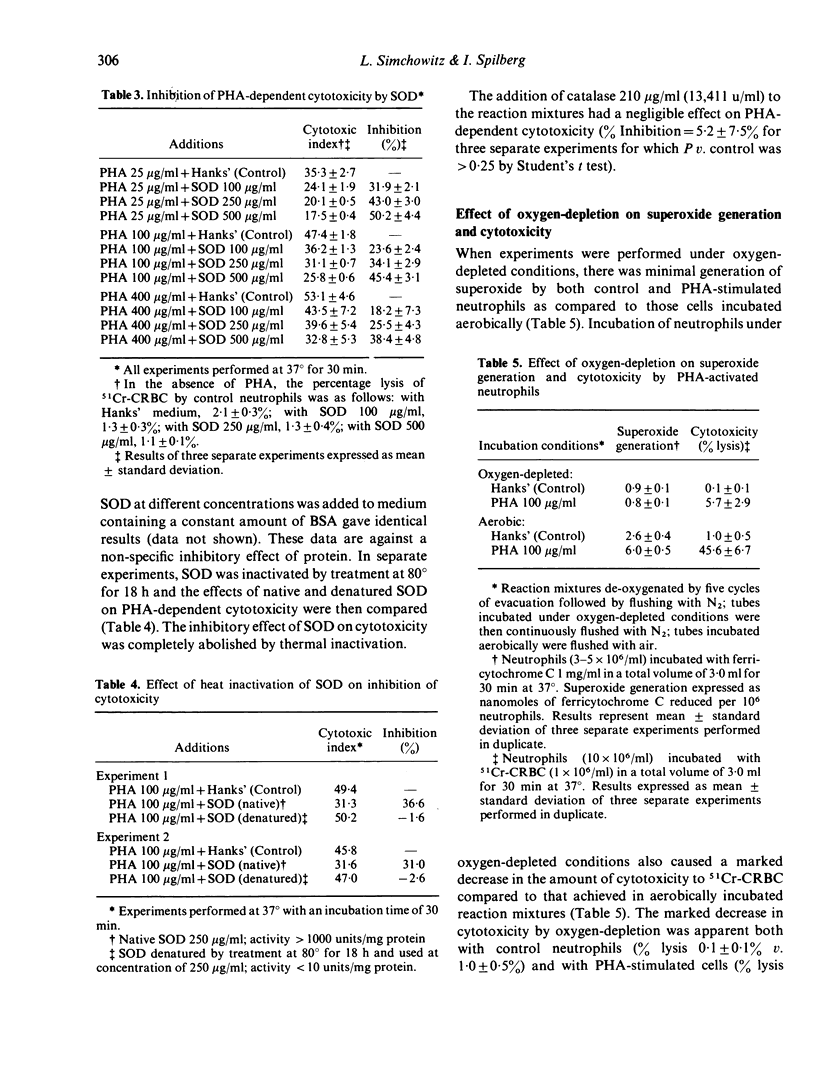
Human peripheral neutrophils became cytotoxic to chicken red blood cells (CRBC) in the presence of lectins as assessed by release of 51chromium from labelled target cells. Phytohaemagglutinin (PHA) and concanavalin A (Con A), which caused time-dependent and dose-dependent cytotoxicity over a concentration range of 25--400 microgram/ml, also caused significant generation of superoxide radicals as measured by ferricytochrome C reduction. Pokeweed mitogen, which does not induce cytotoxicity over the same concentration range, was unable to promote superoxide generation by neutrophils. PHA-induced generation of superoxide paralleled and appeared to precede PHA-dependent cytotoxicity. Superoxide dismutase (SOD), which enzymatically destroys superoxide, caused moderate inhibition of PHA-dependent cytotoxicity over the concentration range of 100--500 microgram/ml whereas catalytically inactive enzyme had no effect. Incubation under oxygen-depleted conditions caused a marked decrease in both PHA-induced superoxide generation and cytotoxicity relative to that obtained with neutrophils incubated aerobically. These findings suggest a central role for superoxide radicals in causing target cell damage in this model of neutrophil-mediated cytotoxicity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs R. T., Karnovsky M. L., Karnovsky M. J. Hydrogen peroxide production in chronic granulomatous disease. A cytochemical study of reduced pyridine nucleotide oxidases. J Clin Invest. 1977 Jun;59(6):1088–1098. doi: 10.1172/JCI108732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Studies on the mechanism of antibody-dependent polymorphonuclear leukocyte-mediated cytotoxicity. J Immunol. 1977 Oct;119(4):1413–1418. [PubMed] [Google Scholar]
- Clark R. A., Olsson I., Klebanoff S. J. Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J Cell Biol. 1976 Sep;70(3):719–723. doi: 10.1083/jcb.70.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee J. A., Teitelbaum H. D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):150–158. doi: 10.1016/0006-291x(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol. 1975 Mar;114(3):1047–1051. [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Soberman R., Goldstein I., Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. doi: 10.1083/jcb.68.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- MacDonald H. R., Bonnard G. D., Sordat B., Zawodnik S. A. Antibody-dependent cell-mediated cytotoxicity: heterogeneity of effector cells in human peripheral blood. Scand J Immunol. 1975 Sep;4(5-6):487–497. doi: 10.1111/j.1365-3083.1975.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Schur P. H. Lectin-dependent neutrophil-mediated cytotoxicity. I. Characteristics. Immunology. 1976 Aug;31(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Schur P. H. Lectin-dependent neutrophil-mediated cytotoxicity. II. Possible mechanisms. Immunology. 1976 Aug;31(2):313–322. [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]
- Zighelboim J., Gale R. P., Kedar E. Polymorphonuclear leukocyte Fc receptors in antibody-dependent cellular cytotoxicity (ADCC). Transplantation. 1976 Jun;21(6):524–526. doi: 10.1097/00007890-197606000-00016. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


