Abstract
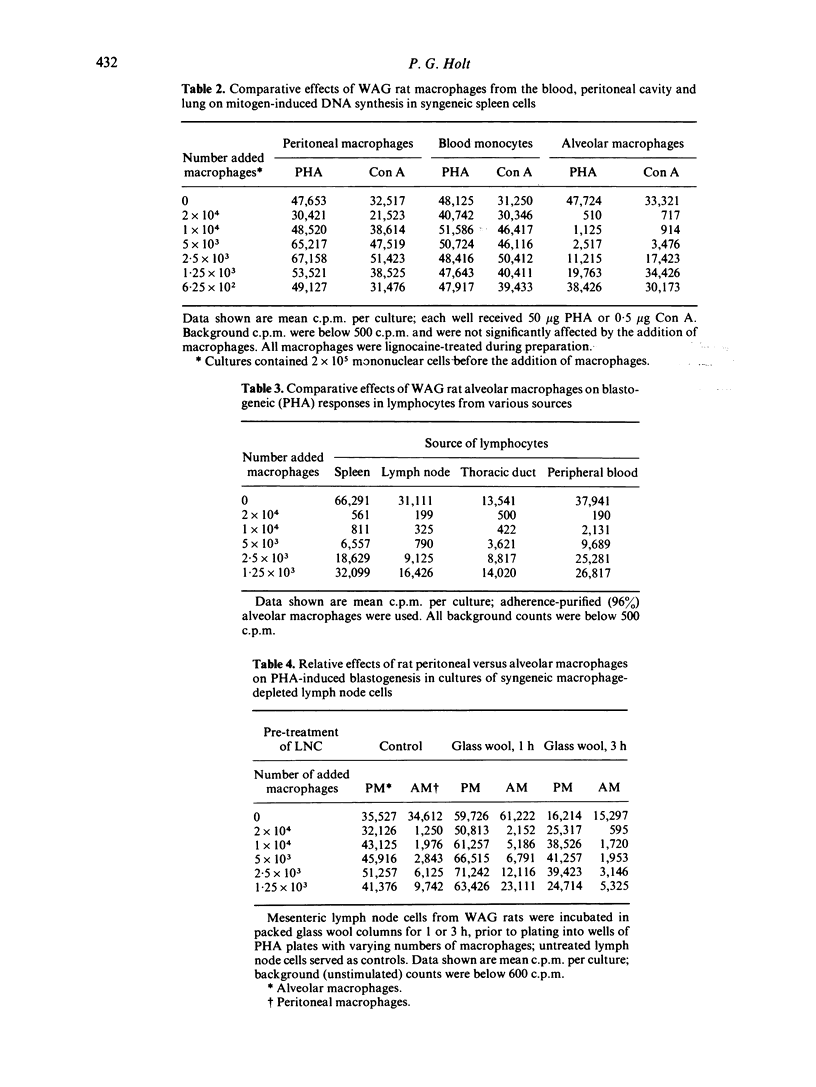
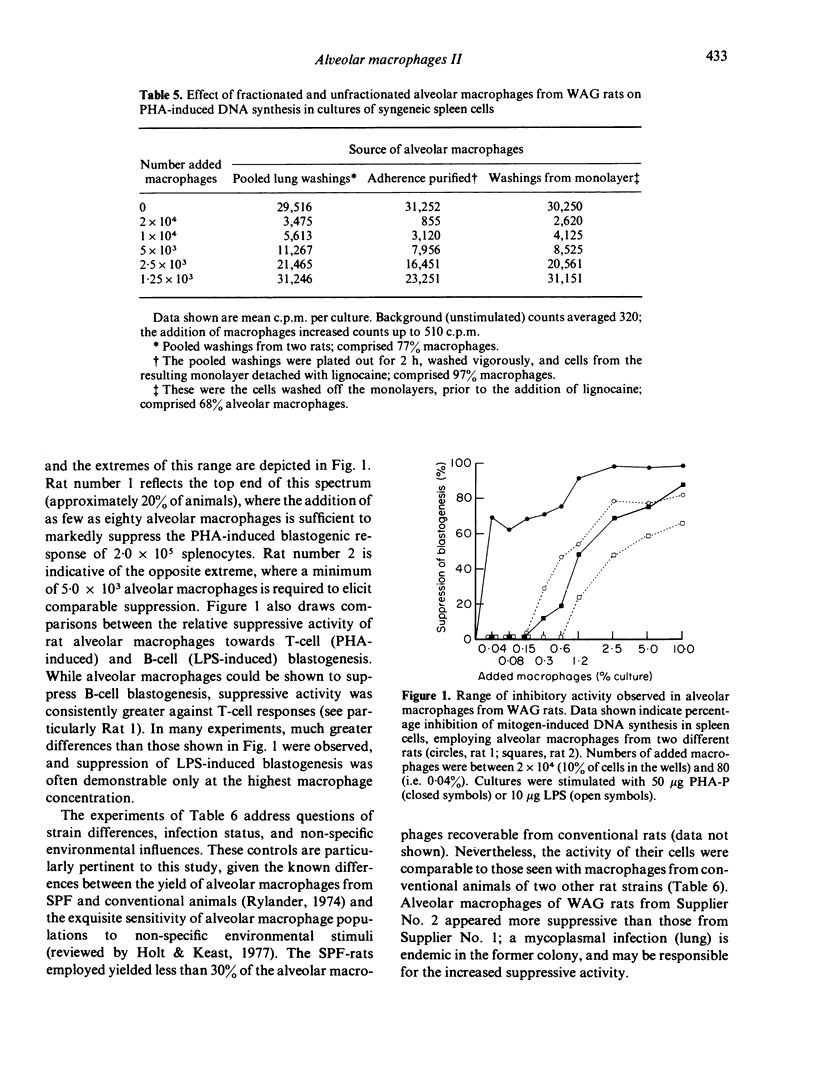
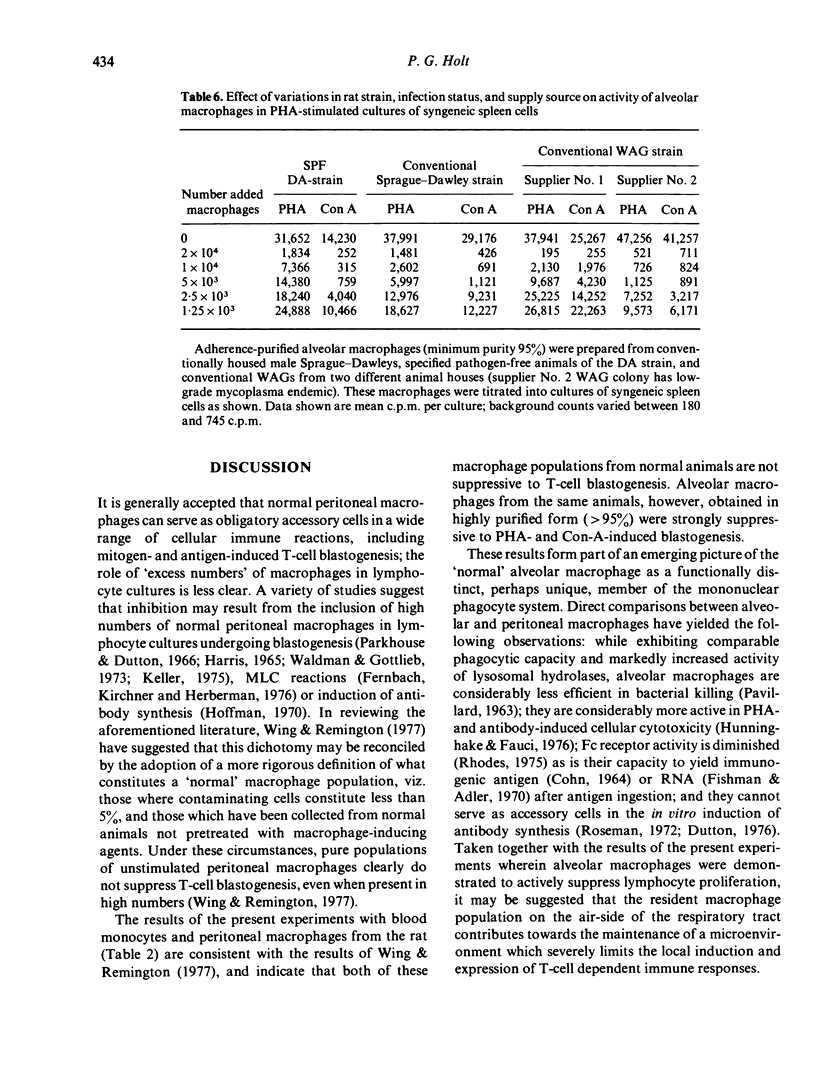
Macrophages were prepared from the lung, peritoneal cavity and blood of normal, unstimulated rats from a number of strains. The macrophages were purified by adherence, and characterized via surface markers, enzyme activity and phagocytic capacity, and subsequently tested for activity in cultures of mitogen-stimulated syngeneic lymphocytes. Peritoneal macrophages and blood monocytes were mildly stimulatory, or ineffective in modulating mitogen-induced DNA synthesis; peritoneal macrophages reconstituted the blastogenic responses of macrophage-depleted lymph node cell cultures to normal limits. In contrast, alveolar macrophages were markedly inhibitory to lymphocyte proliferation; in some instances inhibitory activity was demonstrable when added alveolar macrophages comprised only 0.04% of the total cells in culture. Lymphocyte proliferation induced by T-cell mitogens was more susceptible to this inhibition than was proliferation induced by the B-cell mitogen LPS. Alveolar macrophages recovered from SPF rats, while less in number, exhibited comparable inhibitory activity. These results form part of an emerging picture picture of the normal alveolar macrophage as a potential 'suppressor' of T-cell activity in the lung.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird L. G., Kaplan A. M. Macrophage regulation of mitogen-induced blastogenesis. I. Demonstration of inhibitory cells in the spleens and peritoneal exudates of mice. Cell Immunol. 1977 Jan;28(1):22–35. doi: 10.1016/s0008-8749(77)80003-8. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. THE FATE OF BACTERIA WITHIN PHAGOCYTIC CELLS. 3. DESTRUCTION OF AN ESCHERICHIA COLI AGGLUTINOGEN WITHIN POLYMORPHONUCLEAR LEUCOCYTES AND MACROPHAGES. J Exp Med. 1964 Nov 1;120:869–883. doi: 10.1084/jem.120.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernbach B. R., Kirchner H., Herberman R. B. Inhibition of the mixed lymphocyte culture by peritoneal exudate cells. Cell Immunol. 1976 Mar 15;22(2):399–403. doi: 10.1016/0008-8749(76)90043-5. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol. 1974 Jul;113(1):127–139. [PubMed] [Google Scholar]
- Folch H., Yoshinaga M., Waksman B. H. Regulation of lymphocyte responses in vitro. 3. Inhibition by adherent cells of the T-lymphocyte response to phytohemagglutinin. J Immunol. 1973 Mar;110(3):835–839. [PubMed] [Google Scholar]
- Harris G. Studies of the mechanism of antigen stimulation of DNA synthesis in rabbit spleen cultures. Immunology. 1965 Dec;9(6):529–541. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. III. Studies on the mechanisms of inhibition of T-cell proliferation. Immunology. 1979 Jun;37(2):437–445. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Keast D. Environmentally induced changes in immunological function: acute and chronic effects of inhalation of tobacco smoke and other atmospheric contaminants in man and experimental animals. Bacteriol Rev. 1977 Mar;41(1):205–216. doi: 10.1128/br.41.1.205-216.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung II. Cytotoxic effector function of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):98–104. doi: 10.1016/0008-8749(76)90351-8. [DOI] [PubMed] [Google Scholar]
- Keller R. Major changes in lymphocyte proliferation evoked by activated macrophages. Cell Immunol. 1975 Jun;17(2):542–551. doi: 10.1016/s0008-8749(75)80058-x. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- Nettesheim P., Williams M. L. Induction of the humoral antibody response via the respiratory tract. Ann N Y Acad Sci. 1974;221:220–233. doi: 10.1111/j.1749-6632.1974.tb28222.x. [DOI] [PubMed] [Google Scholar]
- PAVILLARD E. R. In vitro phagocytic and bactericidal ability of alveolar and peritoneal macrophages of normal rats. Aust J Exp Biol Med Sci. 1963 Jun;41:265–274. doi: 10.1038/icb.1963.26. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Dutton R. W. Inhibition of spleen cell DNA synthesis by autologous macrophages. J Immunol. 1966 Nov;97(5):663–669. [PubMed] [Google Scholar]
- Peterson L. B., Thrall R. S., Moore V. L., Stevens J. O., Abramoff P. An animal model of hypersensitivity pneumonitis in the rabbit. Induction of cellular hypersensitivy to inhaled antigens using carrageenan and BCG. Am Rev Respir Dis. 1977 Dec;116(6):1007–1012. doi: 10.1164/arrd.1977.116.6.1007. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Use of the local anesthetic lidocaine for cell harvesting and subcultivation. In Vitro. 1975 Nov-Dec;11(6):379–381. doi: 10.1007/BF02616374. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Macrophage heterogeneity in receptor activity: the activation of macrophage Fc receptor function in vivo and in vitro. J Immunol. 1975 Mar;114(3):976–981. [PubMed] [Google Scholar]
- Rylander R. Influence of infection on pulmonary defense mechanisms. Ann N Y Acad Sci. 1974;221:282–289. doi: 10.1111/j.1749-6632.1974.tb28227.x. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Waldman S. R., Gottlieb A. A. Macrophage regulation of DNA synthesis in lymphoid cells: effects of a soluble factor from macrophages. Cell Immunol. 1973 Oct;9(1):142–156. doi: 10.1016/0008-8749(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]


