Abstract
The mechanisms controlling induction of anergy at the level of naïve CD4+ T cells are poorly understood but thought to reflect limited contact with costimulatory molecules during T cell antigen receptor (TCR) ligation. To clarify this question, naïve TCR transgenic CD4+ cells were exposed to specific peptide presented by transfected antigen-presenting cells (APC) expressing MHC class II molecules with defined accessory molecules. Significantly, culturing CD4+ cells with APC expressing MHC II plus peptide alone elicited early TCR signaling but failed to induce either proliferation or anergy. Culture with APC expressing MHC II plus B7 molecules led to strong proliferation and T cell priming but no anergy. In marked contrast, conspicuous induction of anergy occurred after T cell culture with APC expressing MHC class II and intercellular adhesion molecule-1 (ICAM-1). Thus, at the level of naïve CD4+ cells, anergy induction appears to reflect selective contact with APC expressing ICAM-1 in the absence of B7.
Keywords: rodent, adhesion molecules
The outcome of initial T cell contact with antigen (immunity or tolerance) is important both for assuring adequate responses to infectious pathogens and preventing inappropriate responses to self-antigens. The mechanisms controlling self/nonself discrimination are not fully understood but may hinge on the function of costimulatory molecules. For normal immune responses, it is clear that T cell stimulation involves not only T cell receptor (TCR) recognition of peptide–MHC complexes but also interactions between costimulatory receptors and their ligands on professional antigen-presenting cells (APC). CD28 molecules are the best-characterized costimulatory receptors on T cells. Binding of CD28 to its B7 ligands complements TCR ligation to promote immunological synapses between T cells and APC (1, 2) and ultimately the induction of sustained proliferative responses, cytokine production (e.g., IL-2 and IL-4), and expression of molecules promoting cell survival (e.g., Bcl-xL) (3–8).
When T cells recognize antigen in the absence of costimulation, however, the results are quite different. For primed T cells, TCR stimulation (signal 1) without concomitant coligation of CD28 (signal 2) induces T cell functional unresponsiveness or anergy characterized by the failure to proliferate or make IL-2 on reexposure to antigen presented in the context of optimal costimulation (8–12).
Although this paradigm for anergy induction is well established for T cell clones and lines, there is considerable controversy as to whether delivery of signal 1 alone also induces anergy at the level of naïve CD4+ T cells. In the studies of Lechler and colleagues (13), exposure of unprimed CD4+ T cells to immobilized anti-CD3 mAb was shown to render the surviving cells profoundly unresponsive to subsequent mitogen stimulation. The relevance of this finding to physiological recognition of MHC–peptide on normal APC is somewhat unclear, because the affinity of the TCR for peptide–MHC complexes is more than 1,000-fold lower than the affinity of most antibodies and especially because mAb crosslinking of CD3 can lead to rapid T cell apoptosis (13, 14). Other studies supporting the notion that anergy can be induced in naïve CD4+ cells after exposure to signal 1 alone have relied on the use of transfected mammalian cell lines expressing selected MHC molecules with accessory molecules such as B7 and intercellular adhesion molecule-1 (ICAM-1) (15, 16). Here there is the concern that mammalian cell lines may express a wide variety of endogenous cell surface molecules with the potential to alter T cell responses; additionally, immunomodulatory cytokines and chemokines can be produced by these cell lines (17), thus making it impossible to determine which particular interactions are required for the observed functional outcome. Significantly, circumventing these problems by stimulating naïve TCR transgenic CD4+ cells with immobilized peptide–MHC complexes failed to induce unresponsiveness (18), raising the possibility that something more than signal 1 was required for anergy induction.
To seek comprehensive information on anergy induction in naïve T cells, we used transfected insect cells as APC for TCR transgenic CD4+ cells. The advantage of Drosophila (Dros) cells as APC is that these cells lack mammalian costimulatory molecules and do not produce mammalian cytokines, chemokines, or soluble receptors. Dros cell lines transfected with murine class II molecules with or without additional accessory molecules such as ICAM-1 and/or B7.2 were used to present peptide to class II-restricted D011 TCR transgenic CD4+ T cells. This approach allowed us to analyze how specific accessory molecules influence the outcome of TCR peptide recognition.
Materials and Methods
Mice.
D011 TCR transgenic mice (kindly provided by K. Murphy and D. Loh, Washington University School of Medicine, St. Louis) were bred at the Scripps Research Institute. BALB/cJ mice were obtained from The Jackson Laboratory.
Dros Cell Lines.
Dros cell lines expressing murine MHC H2-Ad molecules +/− murine ICAM-1 and/or B7.2 molecules were generated as described (19, 20). Briefly, cDNA clones for B7.2, ICAM-1, and H2-Ad α and β chains were expressed in Dros Schneider SC2 cells under the control of a metallothionein promoter. Cell lines expressing the desired proteins were selected by growing in Schneider's Dros medium (GIBCO/BRL) containing 5% FCS, antibiotics, and 500 μg/ml geneticin as well as by several rounds of cell sorting. Forty-eight hours before use in experiments, expression of the transfected genes is induced by addition of CuSO4 (1 mM).
Cell Purification.
CD4+ T cells were purified from pooled lymph nodes as described (20). Lymph node cell suspensions were passed over nylon wool columns followed by antibody and C′-mediated killing of residual cells bearing CD8 (3.168 Ab), H2-Ad (MKD6 Ab), H2-E (14–44 Ab), or heat stable antigen (HSA) (J11d Ab). In some experiments, CD4+ T cells were further purified by depletion of CD8 and class II-positive cells by using Dynal (Oslo) magnetic beads and separation on a stepwise Percoll density gradient (Amersham Pharmacia) to enrich for small resting T cells. Both approaches gave similar results.
Spleen APC were prepared by depletion of T cells by treatment with anti-T cell antibody (anti-CD8, 3.168; anti-CD4, RL-172 Ab; and anti-Thy 1, J1j Ab) and C′.
Proliferation Assays.
CD4+ T cells purified as above were cultured in microtiter wells at a concentration of 4–5 × 104 per well with 2 × 105 Dros APC or 5 × 105 mitomycin C-treated T-depleted spleen APC and the indicated concentration of ovalbumin (ova) peptide (). Culture medium was RPMI medium 1640 supplemented with 10% FCS/5% NCTC 109/5 × 10−5 M 2-mercaptoethanol/glutamine/Hepes buffer/antibiotics. Culture wells were pulsed with 1 μCi [3H]thymidine 12–18 h before harvesting on glass fiber filters and counting in an EG & G (Turku, Finland) Wallac Microbeta TriLux scintillation counter. All cultures were set up in triplicate.
Dros cell lines, which are propagated at 25°C, die within ≈24 h of culture at 37°C, thus excluding any contribution of Dros cell division to the proliferative responses measured.
ELISA Assays.
Culture supernatant was removed from the above cultures before pulsing with [3H]thymidine for analysis of cytokine accumulation. Cytokines were measured by using a sandwich ELISA assay as described (20). Both capture antibodies and biotinylated detection antibodies were obtained from PharMingen; recombinant cytokines used for construction of standard curves were either from Genzyme or PharMingen.
Anergy Assays.
Naïve CD4+ T cells purified as above were cultured with the indicated H2-Ad-expressing transfected Dros APC and ova peptide in 5 ml of complete culture medium. At 24 h, the Dros cells and peptide were removed by passage over 40% Percoll gradients and the washed CD4+ cells were recultured in fresh RPMI medium 1640 complete culture medium for an additional 3 days. At that time, viable cells were enumerated and assayed in a typical proliferation assay for their response to a titration of ova peptide presented by T-depleted, mitomycin C-treated spleen APC.
Flow Cytometry.
Approximately 1 × 106 viable cells were incubated with the indicated antibodies which had been previously titrated to determine the optimal concentration for staining. Incubations were for 20 min and the cells were washed twice between incubations with PBS containing 2.5% heat-inactivated, γ-globulin-free horse serum and 1% NaN3. Dead cells were excluded by the addition of propidium iodide and generally data on 10,000 viable cells were collected by using either a FACsCalibur, FACSort, or FACscan instrument; data were analyzed by using winmdi software (Joe Trotter, The Scripps Research Institute).
Immunoprecipitations and Western Blots.
Equivalent numbers of purified CD4+ T cells were mixed with the indicated ova peptide prepulsed APC for 5 min. Cell lysates were prepared by using lysis buffer containing 20 mM Tris/150 mM NaCl/200 μM sodium vanadate/2 mM EDTA/50 mM NaF/0.5% Triton X-100/1 mM PMSF. After preclearing with Pansorbin (Calbiochem), immunoprecipitates were collected on Pansorbin precoated with hamster anti-CD3ɛ Ab (145–2C11) and resolved on 12.5% SDS/PAGE gels under reducing conditions. Proteins were transferred to Immobilon P membranes (Millipore), which were incubated with biotin-conjugated 4G10 antiphosphotyrosine antibody (Upstate Biotechnology, Lake Placid, NY) followed by SA-horseradish peroxidase (Amersham International) and developed by using chemiluminescence (NEN). Films were scanned by using a Molecular Dynamics scanning densitometer and images were analyzed by using imagequant software (Molecular Dynamics).
Results
Experimental Approach.
As a source of APC, Dros cells were transfected with murine class II molecules along with ICAM-1 and/or B7.2 and used to present ova peptide 323–339 to class II-restricted, ova-reactive D011 TCR transgenic CD4+ cells (20). Although Dros cell DNA is mitogenic for mammalian APC (21), we have seen no evidence to indicate that culture with Dros cells alone has any detectable effect on rigorously purified CD4+ cells. The efficacy of this approach has now been well documented (19, 20).
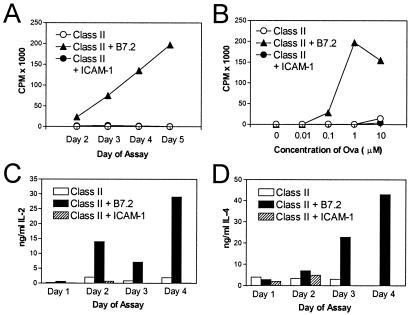
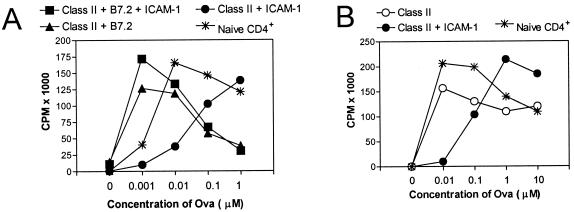
As reported (20) and illustrated in Fig. 1A, Dros APC-expressing class II H2-Ad molecules in the absence of costimulatory molecules (hereafter referred to as H2-Ad Dros APC) failed to induce detectable proliferative responses by highly purified naïve D011 CD4+ cells. By contrast, expression of the CD28/CTLA-4 ligand B7.2 on H2-Ad Dros APC stimulated strong dose-dependent proliferative responses (Fig. 1 A and B). When H2-Ad Dros APC expressing ICAM-1 without B7.2 were used as APC, D011 CD4+ cells failed to exhibit significant [3H]thymidine uptake regardless of the day of assay or the concentration of peptide (Fig. 1 A and B). Similarly, stimulation with ICAM-1-expressing H2-Ad Dros APC failed to induce significant production of IL-2 or IL-4 as detected by measuring the accumulation of cytokines in the culture medium by ELISA (Fig. 1 C and D). By contrast, B7.2-expressing Dros APC induced strong production of both of these cytokines. Expression of both B7 and ICAM-1 on APC led to strong proliferative responses and IL-2 production, although IL-4 was consistently reduced (data not shown). This latter result supports previous studies by ourselves and others showing that naïve CD4+ cells are able to produce IL-4 via CD28 costimulation (20, 22–25) but only in the absence of ICAM interactions that down-regulate IL-4 production in primary cultures (20, 26).
Figure 1.
Expression of B7.2 but not ICAM-1 promotes strong CD4+ T cell proliferative responses and cytokine production. D011 CD4+ cells purified as described in Materials and Methods were cultured with ova peptide and class II H2-Ad-positive Dros APC with the indicated costimulatory molecules. [3H]thymidine uptake of triplicate cultures was measured on days 2–5. Supernatant was removed before addition of [3H]thymidine for analysis of cytokine production by ELISA. (A) Kinetics of the proliferative response to 1 μM ova peptide. (B) Dose–response curve of D011 CD4+ cells stimulated with the indicated concentrations of ova peptide presented by Dros APC expressing the indicated murine accessory molecules. The response shown is that measured on day 5, the peak response in this particular experiment. (C and D) IL-2 and IL-4 accumulation in the above cultures was assessed on days 1–4; the peak IL-2 and IL-4 levels in the culture supernatant were observed with 10 μM ova, which is illustrated.
Early Indicators of CD4+ T Cell Activation.
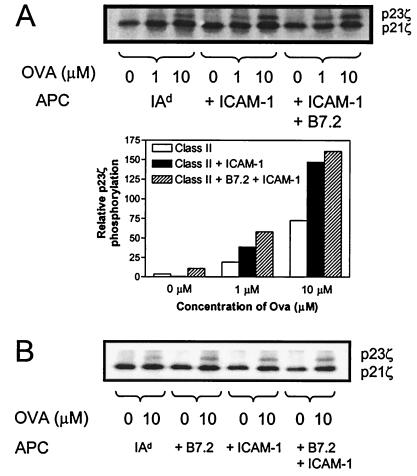
To determine whether D011 CD4+ cells simply ignored peptide presented by ICAM+ B7− APC, several early indicators of T cell activation were examined. Among the earliest events in TCR-mediated signaling is the activation of src family kinases which phosphorylate ITAM motifs within the cytoplasmic tails of CD3 and ζ chains (27–30). Because TCR signaling alone should be sufficient for ζ chain phosphorylation, we tested whether recognition of peptide presented by H2-Ad Dros APC +/− ICAM-1 led to phosphorylation of TCR ζ chains. D011 CD4+ cells were stimulated with ova peptide presented by H2-Ad Dros APC lacking accessory molecules or coexpressing ICAM-1 and/or B7.2 (Fig. 2A). Anti-CD3 immunoprecipitates of cell lysates of these samples were analyzed by Western blotting by using antiphosphotyrosine antibody to assess associated ζ chain phosphorylation. In the absence of ova peptide, only the faster migrating form of phosphorylated ζ was apparent, irrespective of which APC was used, thereby confirming the requirement for peptide in this system. With ova peptide, the slower migrating p23-phosphorylated form of ζ was detected with all three APC, consistent with the prevailing view that TCR signaling alone is sufficient for initiating ζ chain phosphorylation. Quantitation by densitometry showed, nonetheless, that tyrosine phosphorylation of p23ζ was increased significantly after stimulation with H2-Ad APCs expressing ICAM-1 and/or B7.2 (Fig. 2 A and B). These findings confirm that TCR occupancy alone is sufficient to initiate TCR signaling in this system, although ζ chain phosphorylation is enhanced by accessory molecule interactions.
Figure 2.
Coexpression of ICAM-1 promotes the appearance of the hyperphosphorylated p23 form of ζ. D011 CD4+ cells were stimulated for 5 min with the indicated concentration of ova peptide presented by Dros APC-expressing class II alone (IAd), class II plus ICAM-1 (+ ICAM-1), or class II plus ICAM-1 plus B7.2 (+ ICAM-1 + B7.2). Cell lysates were immunoprecipitated with anti-CD3ɛ and the precipitated proteins were resolved on SDS/PAGE. After transfer, phosphorylated ζ was detected by blotting with antiphosphotyrosine (4G10) Ab. The p23ζ bands in the above blot were quantitated by densitometry and the relative volume was plotted. (B) A second experiment including Dros APC expressing B7.2.
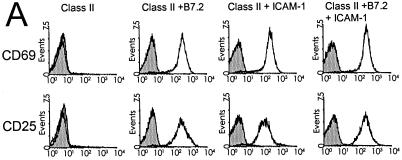
Activation of CD4+ T cells also is associated with the early up-regulation of several cell surface molecules including CD69 and CD25. To determine whether ICAM-1 costimulates TCR signaling as manifested by the expression of these two proteins, D011 CD4+ cells were cultured overnight with ova peptide and H2-Ad Dros APC expressing ICAM-1 and/or B7.2. Expression of CD69 and CD25 was examined at ≈24 h by using flow cytometry. As illustrated in Fig. 3, stimulation of naïve CD4+ cells with peptide presented by H2-Ad Dros APC expressing either B7.2 or ICAM-1 induced marked up-regulation of both of these cell surface proteins in a dose-dependent fashion (data not shown). In contrast, culture with H2-Ad Dros APC (not expressing costimulatory molecules) failed to induce either CD69 or CD25 expression.
Figure 3.
Both B7 and ICAM-1 costimulate up-regulation of CD69 and CD25. Purified D011 CD4+ cells were cultured for ≈24 h with ova peptide and Dros APC expressing the indicated accessory molecules. CD69 and CD25 expression was assessed by flow cytometry electronically gating on CD4+ T cells and excluding dead cells by using propidium iodide. D011 CD4+ cells were cultured with no ova peptide (shaded histograms) or with 1 μM ova peptide (open histograms) and the indicated Dros APC.
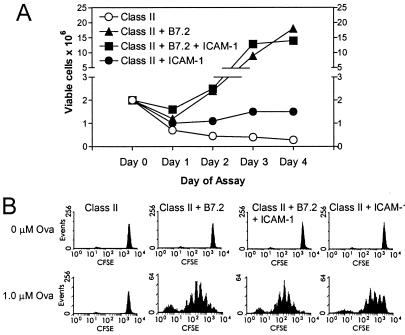
In addition to up-regulating activation markers, ICAM-1-costimulated CD4+ cells increased significantly in cell size as indicated by changes in their forward scatter characteristics (data not shown) and also exhibited an increase in viable cell recoveries when compared with cells cultured with peptide and Dros APC-expressing class II alone (Fig. 4A). Indeed, analysis of cell division by monitoring 5,6-carboxyfluorescein diacetate succinimyl ester (CFSE) staining indicated that despite the lack of significant [3H]thymidine uptake by ICAM-1-stimulated cells (Fig. 1), there was some degree of cell division, although much less than with B7+ APC (Fig. 4B). Thus, by these various parameters, costimulation of naïve CD4+ cells via ICAM-1 clearly leads to activation of CD4+ cells as suggested previously (15, 31). Yet these cells fail to expand extensively or synthesize significant quantities of cytokines. Thus, T cell activation via ICAM-1 may be considered partial.
Figure 4.
(A) Viable cell counts after culture of D011 CD4+ cells with H2-Ad Dros APC expressing the indicated costimulatory molecules. Purified D011 CD4+ cells (2 × 106) were cultured with Dros APC and 1 μM ova peptide in 5-ml cultures. On the indicated day of assay, viable cell counts were determined by phase microscopy. (B) D011 CD4+ cells were labeled with CFSE as described before culture as above with Dros APC expressing the indicated accessory molecules. The results shown were analyzed on day 3 of culture.
Influence of LFA-1–ICAM-1 Priming on Secondary Responses.
A variety of studies in other systems suggest that partial activation of CD4+ cells, i.e., activation that fails to lead to IL-2 production, can have profound effects on the subsequent responsiveness of these cells when restimulated with peptide presented by APC bearing a full complement of costimulatory/accessory molecules (32). To determine whether ICAM-1-mediated costimulation of naïve CD4+ cells induces either priming or tolerance, D011 CD4+ cells were cultured overnight with ova peptide and H2-Ad Dros APC expressing ICAM-1 alone, B7.2 alone, or ICAM-1 plus B7.2. The Dros APC and peptide were removed at 24 h and the cells were allowed to rest in fresh culture medium for 3 days before restimulation with peptide and spleen APC. The subsequent proliferative response was measured on days 2–5.
As illustrated in Fig. 5A, primary stimulation of naïve CD4+ cells with B7.2+ or B7.2 plus ICAM-1+ H2-Ad Dros APC led to efficient priming, i.e., a marked reduction (≈10×) in the concentration of peptide required for peak secondary proliferative responses to peptide-pulsed spleen APC as compared with naïve unprimed CD4+ cells. In contrast, exposure to peptide presented by Dros APC expressing ICAM-1 in the absence of B7.2 raised the threshold for optimal responses by 10- to 100-fold [depending on the particular experiment and the concentration of peptide in the priming culture (data not shown)]. These results, which are representative of 10 separate experiments, indicate that “priming” CD4+ cells by ICAM+ B7− APC induced strong functional anergy. Analysis of TCR and CD4 levels on the day of restimulation ruled out the possibility that TCR/CD4 down-regulation accounted for the hyporesponsiveness observed here because expression was comparable on ICAM-1 stimulated vs. B7 plus ICAM-1-stimulated T cells (data not shown). It is important to emphasize that the hyporesponsiveness of ICAM-1-primed CD4+ cells does not reflect an irreversible commitment to cell death because these cells do respond well to high concentrations of ova peptide.
Figure 5.
Priming with B7.2-expressing APC lowers the threshold for secondary responses whereas priming with ICAM-1 raises this threshold. Purified CD4+ D011 T cells were primed by 24-h culture with 1 μM ova peptide presented by H2-Ad Dros APC expressing the indicated accessory molecules. Dros APC were removed after 24 h and the primed cells rested in fresh complete medium for 3 days before restimulation. Equal numbers of viable primed and freshly isolated “Naïve” D011 cells were restimulated with mitomycin C-treated T-depleted splenic APC and the indicated concentration of ova peptide. [3H]thymidine uptake of triplicate cultures was measured on days 2–5 and the peak response (day 4) is illustrated. A and B represent two distinct experiments.
Significantly, priming CD4+ cells in the presence of B7.2− ICAM-1− APC, i.e., APC expressing only class II molecules + peptide, generally had little effect on the threshold of activation, although the responses were sometimes somewhat lower than those of naïve CD4+ cells (Fig. 5B and see Fig. 7B). Given the typically poor viability of these cells on the day of assay (Fig. 4), it is quite likely that these lower responses simply reflected the overall poor health of the surviving T cells.
Figure 7.
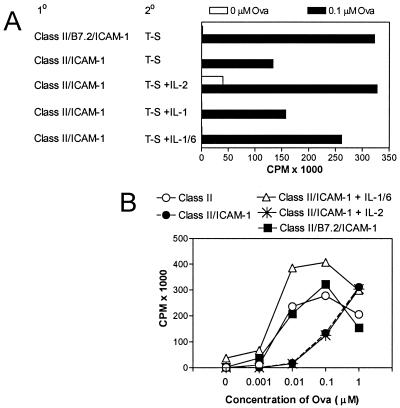
Cytokines modulate induction and extent of hyporesponsiveness. (A) Naïve D011 CD4+ cells were cultured for 24 h with the indicated Dros APC and 1 μM ova peptide without added cytokines. After removal of APC at 24 h and 3 days rest, equal numbers of viable cells were restimulated with ova peptide presented by splenic APC. Secondary responses at 0.1 μM are shown. In some cultures, exogenous IL-2 (20 units/ml), IL-1 (20 units/ml), or IL-1 plus IL-6 (60 units/ml) was added to the secondary culture. (B) Naïve D011 CD4+ cells were primed for 24 h by culture with Dros APC expressing the indicated accessory molecules in the presence of cytokines. After 24 h, the cells were washed and rested for 3 days before restimulation with ova peptide and splenic APC in the absence of cytokines.
Cytokines Produced After Secondary Stimulation of ICAM-1- vs. B7.2-Primed CD4+ Cells.
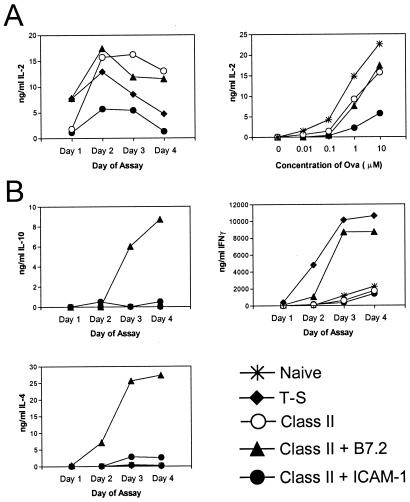
The above data indicated that high concentrations of peptide were able to overcome the hyporesponsiveness of ICAM-1-primed CD4+ cells for proliferative responses. To determine whether cytokine production was similarly restored by high antigen concentration, we measured IL-2 production during the secondary response of ova-primed CD4+ cells to ova peptide/spleen APC. Primary stimulation of naïve CD4+ cells with ova presented by either T-depleted spleen APC, H2-Ad Dros APC, or B7.2+ H2-Ad Dros APC generated primed cells that produced significant levels of IL-2 during the secondary response; the highest concentration of IL-2 in the culture supernatants was seen at 10 μM ova peptide, which is illustrated in Fig. 6A. Priming with H2-Ad Dros APC expressing ICAM-1 without B7 generated cells that produced much smaller quantities of IL-2 and that required high concentrations of peptide in culture (Fig. 6 A and B). Thus, for IL-2 production, high concentrations of peptide only partially restored the ability of ICAM-1-primed cells to produce IL-2.
Figure 6.
Cytokine production during secondary responses of primed D011 CD4+ cells. Purified D011 CD4+ cells were cultured as in Fig. 5. Equal numbers of viable primed cells or freshly isolated “Naïve” D011 cells were restimulated with T-depleted spleen APC and ova peptide. Supernatant from the secondary cultures was removed at daily intervals and analyzed for the indicated cytokines by ELISA. Symbols indicate the type of APC used in the primary culture. (Naïve, unprimed CD4+; T-S, T-depleted spleen APC; class II, IAd+ Dros APC; class II plus B7.2, IAd + B7.2+ Dros APC; class II plus ICAM-1, IAd + ICAM-1+ Dros APC.) (A Left) The kinetics of IL-2 production with 10 μM ova peptide is shown, and (Right) the dose–response curves are illustrated (day 3). (B) Maximum production of IL-10, IFN-γ, and IL-4 was observed with 10 μM ova peptide in the secondary culture; these results are illustrated here.
Examination of other cytokines such as IL-10, IFN-γ, and IL-4 suggested that ICAM-1-primed cells had a generalized defect in cytokine production (Fig. 6B). Thus, whereas B7.2-primed cells produced large quantities of all of these cytokines, ICAM-1-primed cells produced barely detectable quantities of any of these cytokines regardless of the day of assay (days 1–5) or the concentration of ova used for stimulation.
Influence of Exogenously Added Cytokines on the Induction and Maintenance of Anergy.
Previous studies examining anergic T cell clones and lines showed that provision of exogenous IL-2 restored proliferative responses (9, 32). To determine whether IL-2 had similar effects on hyporesponsive ICAM-1-primed cells, anergic D011 CD4+ cells were restimulated with peptide presented by splenic APC with or without exogenously added cytokines. Addition of IL-2 restored secondary proliferative responses induced by low concentrations of ova peptide to levels similar to those observed after stimulation with Dros APC coexpressing ICAM-1 with B7.2 (Fig. 7A). Interestingly, although addition of IL-1 alone did little to promote the response of ICAM-1-primed cells, a combination of both IL-1 and IL-6 also largely restored maximal proliferative responses.
To determine whether cytokines also control the induction of anergy in naïve CD4+ cells, exogenous IL-2 or IL-1 plus IL-6 was added during the initial 24 h priming culture. In contrast to the effect of IL-2 in overcoming the hyporesponsiveness of ICAM-1-primed anergic T cells, addition of IL-2 during the 24-h priming phase did not significantly interfere with the induction of anergy (Fig. 7B). Overcoming the induction of anergy with IL-2 required its presence during both priming and the subsequent 3-day rest period (data not shown). Interestingly, in contrast to IL-2, the presence of IL-1 and IL-6 during the initial 24-h priming culture did prevent the induction of anergy. This finding is consistent with data showing that both IL-1 and IL-6 provide costimulation for naïve D011 CD4+ cells cultured with peptide presented by class II+ Dros APC expressing ICAM-1 (data not shown). In these primary proliferative responses, the effects of IL-1 and IL-6 on proliferation are additive.
Discussion
The findings here clarify the requirements for induction of anergy in naïve CD4+ T cells. In contrast with several earlier studies (13, 16), subjecting naïve CD4+ cells to signal 1 alone, i.e., to peptide-pulsed class II+ APC devoid of mammalian accessory molecules, led to early TCR-mediated signaling events such as ζ chain phosphorylation, but failed to activate proliferative responses or render the cells anergic. This result is in agreement with the observation of Sagerstrom et al. (18) that exposing purified naïve CD4+ cells to immobilized peptide–MHC complexes failed to induce unresponsiveness.
Our findings extend these studies by showing that T cells displayed functional ignorance to cell-bound MHC–peptide complexes displayed on living cells. In addition, we show that in marked contrast to T cell exposure to MHC–peptide complexes alone, coengagement of LFA-1 during TCR recognition of peptide–MHC complexes promotes the induction of conspicuous hyporesponsiveness (anergy), as manifested by decreased sensitivity of the cells to subsequent restimulation with peptide presented by professional APC exhibiting a full range of accessory molecule ligands. Although the biochemical mechanisms by which ICAM-1 augments hyporesponsiveness during priming of naïve T cells are not yet defined, the capacity to promote partial CD4+ cell activation is likely to be a key factor. Thus, although peptide-mediated stimulation of D011 CD4+ cells with accessory molecule-deficient APC leads to early TCR signaling, no downstream evidence for activation was observed. ICAM-1 expressing APC, in contrast, induced blastogenesis, expression of activation markers such as CD69, CD40L, and CD25, and some cell division. The model we favor is that, as for cell lines (32), partial activation is required for anergy induction in unprimed CD4+ cells; unlike cell lines, where signal 1 alone can induce partial activation, naïve CD4+ cells are generally not activated by signal 1 alone. Additional signaling via accessory molecules is required to achieve partial activation. Although we have studied only ICAM-1, other accessory molecules might also promote hyporesponsiveness, e.g., other integrins and their ligands. The types of molecules involved, though, are likely to be limited. Thus, testing CD27, one of the weakly costimulatory members of the tumor necrosis factor receptor family, revealed that although primary peptide-dependent proliferative responses were poorly induced by CD27 ligation, the surviving cells were fully functional on secondary stimulation (M. C. Walsh, E. Chronpoulou, L.K., P.A.P., and S.R.W., unpublished work). With regard to B7, it should be emphasized that, even with a wide range of peptides, we failed to see anergy induction with B7+ APC.
How ICAM-1 mediates its anergy-promoting effects is not clear. One possibility is that anergy simply reflects a strong signal 1. ICAM-1 interaction with LFA-1 facilitates adhesion, thereby augmenting TCR contact with MHC–peptide and thus intensifying signal 1. The studies reporting anergy in T cells stimulated with anti-CD3 mAb, which presumably delivers a very strong signal 1, are at face value consistent with this possibility (13). Nonetheless, even very high concentrations of peptide (up to 1,000-fold higher than that required for stimulation) failed to overcome the requirement for ICAM-1 to induce partial activation and/or anergy in D011 CD4 cells, suggesting that increased ligand density alone is not sufficient. An alternative role for ICAM-1, which is not mutually exclusive, is that binding to LFA-1 induces a qualitatively distinct signal 2, which, in conjunction with signal 1, causes the cells to enter a refractory (anergic) state. This idea is difficult to assess because, although signaling via integrins is well documented, information on LFA-1 signaling in T cells is very limited.
Irregardless of the mechanisms involved, the present data show that selective contact of T cells with ICAM-1 plus MHC–peptide during priming leads to a prominent hyporesponsive state. With regard to in vivo relevance, a number of studies have suggested that antigen presentation by resting B cells causes T cell anergy instead of priming (33, 34). This finding is of particular interest because resting B cells express high levels of ICAM-1 but minimal levels of B7 (35). Even for dendritic cells, it is notable that immature subsets can express low levels of B7 with significant levels of ICAM-1 (36, 37). These findings correlate with the observation that T cell priming in vivo in the absence of adjuvants is often abortive and leads to anergy induction (38). As suggested elsewhere (39, 40), adjuvants may function in part by up-regulating B7 on APC; thus, adjuvants convert anergy-inducing B7lo ICAM-1+ APC to immunogenic B7hi ICAM-1+ APC.
The function of adjuvants also may be relevant to our finding that, unlike IL-2, addition of IL-1 and IL-6 during initial culture of T cells with B7− ICAM-1+ APC prevented anergy induction, and instead led to strong priming. This finding is in agreement with reports that IL-1 and IL-6 provide costimulation for resting T cells (17, 41–43). Because adjuvants can stimulate synthesis of cytokines by APC (44–46), adjuvants may prevent anergy induction not only by causing B7 up-regulation but also by stimulating resting APC to synthesize IL-1 and IL-6; with these cytokines, even B7lo ICAM-1+ APC would be immunogenic.
Acknowledgments
This work was supported by Grants CA41993, CA25803, and AI39664 from the U.S. Public Health Service, and grants from the Juvenile Diabetes Foundation and R. W. Johnson Pharmaceutical Research Institute. This is The Scripps Research Institute Publication No. 12775-IMM.
Abbreviations
- Dros
Drosophila
- TCR
T cell antigen receptor
- LFA-1
lymphocyte function-associated antigen 1
- APC
antigen-presenting cells
- ICAM-1
intercellular adhesion molecule-1
- ova
ovalbumin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011397798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011397798
References
- 1.Wulfing C, Davis M. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 2.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 3.Linsley P S, Ledbetter J A. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 4.Allison J P. Curr Opin Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins M K. Immunity. 1994;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 6.June C H, Ledbetter J A, Linsley P S, Thompson C B. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 8.Powell J D, Ragheb J A, Kitagawa-Sakakida S, Schwartz R H. Immunol Rev. 1998;165:287–300. doi: 10.1111/j.1600-065x.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 9.Mueller D L, Jenkins M K, Schwartz R H. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 10.Harding F A, McArthur J G, Gross J A, Raulet D H, Allison J P. Nature (London) 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 11.Tan P, Anasetti C, Hansen J A, Melrose J, Brunvand M, Bradshaw J, Ledbetter J A, Linsley P S. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimmi C D, Freeman G J, Gribben J G, Gray G, Nadler L M. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai J G, Bartok I, Chandler P, Vendetti S, Antoniou A, Dyson J, Lechler R. Eur J Immunol. 1999;29:686–692. doi: 10.1002/(SICI)1521-4141(199902)29:02<686::AID-IMMU686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto H, Sprent J. J Immunol. 1999;163:1817–1826. [PubMed] [Google Scholar]
- 15.Zuckerman L A, Pullen L, Miller J. J Immunol. 1998;160:3259–3268. [PubMed] [Google Scholar]
- 16.Boussiotis V A, Freeman G J, Gray G, Gribben J, Nadler L M. J Exp Med. 1993;178:1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sepulveda H, Cerwenka A, Morgan T, Dutton R W. J Immunol. 1999;163:1133–1142. [PubMed] [Google Scholar]
- 18.Sagerstrom C G, Kerr E M, Allison J P, Davis M M. Proc Natl Acad Sci USA. 1993;90:8987–8991. doi: 10.1073/pnas.90.19.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Z, Brunmark A, Jackson M R, Loh D, Peterson P A, Sprent J. Proc Natl Acad Sci USA. 1996;93:14736–14741. doi: 10.1073/pnas.93.25.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luksch C R, Winqvist O, Ozaki M E, Karlsson L, Jackson M R, Peterson P A, Webb S R. Proc Natl Acad Sci USA. 1999;96:3023–3028. doi: 10.1073/pnas.96.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun S, Zhang X, Tough D F, Sprent J. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranger A M, Das M P, Kuchroo V K, Glimcher L H. Int Immunol. 1996;8:1549–1560. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 23.Gause W, Urban J, Linsley P, Lu P. Immunol Res. 1995;14:176–178. doi: 10.1007/BF02918215. [DOI] [PubMed] [Google Scholar]
- 24.Gause W C, Halvorson M J, Lu P, Greenwald R, Linsley P, Urban J F, Finkelman F D. Immunol Today. 1997;18:115–120. doi: 10.1016/s0167-5699(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 25.Rulifson I C, Sperling A I, Fields P E, Fitch F W, Bluestone J A. J Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 26.Salomon B, Bluestone J A. J Immunol. 1998;161:5138–5142. [PubMed] [Google Scholar]
- 27.Weiss A, Littman D R. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 28.Cantrell D. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 29.Wange R L, Samelson L E. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 30.Germain R N, Stefanova I. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 31.van Seventer G A, Semnani R T, Palmer E M, McRae B L, van Seventer J M. Transplant Proc. 1998;30:4270–4274. doi: 10.1016/s0041-1345(98)01410-9. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz R H. J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eynon E E, Parker D C. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs E J, Matzinger P. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 35.Hathcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razi-Wolf Z, Falo L D, Jr, Reiser H. Eur J Immunol. 1994;24:805–811. doi: 10.1002/eji.1830240405. [DOI] [PubMed] [Google Scholar]
- 37.Larsen C P, Ritchie S C, Pearson T C, Linsley P S, Lowry R P. J Exp Med. 1992;176:1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 39.Khoruts A, Mondino A, Pape K A, Reiner S L, Jenkins M K. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparwasser T, Koch E S, Vabulas R M, Heeg K, Lipford G B, Ellwart J W, Wagner H. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Lotz M, Jirik F, Kabouridis P, Tsoukas C, Hirano T, Kishimoto T, Carson D A. J Exp Med. 1988;167:1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holsti M A, Raulet D H. J Immunol. 1989;143:2514–2519. [PubMed] [Google Scholar]
- 43.Stein P H, Singer A. Int Immunol. 1992;4:327–335. doi: 10.1093/intimm/4.3.327. [DOI] [PubMed] [Google Scholar]
- 44.Brewer J M, Alexander J. Cytokines Cell Mol Ther. 1997;3:233–246. [PubMed] [Google Scholar]
- 45.Klinman D M, Verthelyi D, Takeshita F, Ishii K J. Immunity. 1999;11:123–129. doi: 10.1016/s1074-7613(00)80087-4. [DOI] [PubMed] [Google Scholar]
- 46.Foss D L, Murtaugh M P. Adv Vet Med. 1999;41:83–104. doi: 10.1016/s0065-3519(99)80010-x. [DOI] [PubMed] [Google Scholar]