Abstract
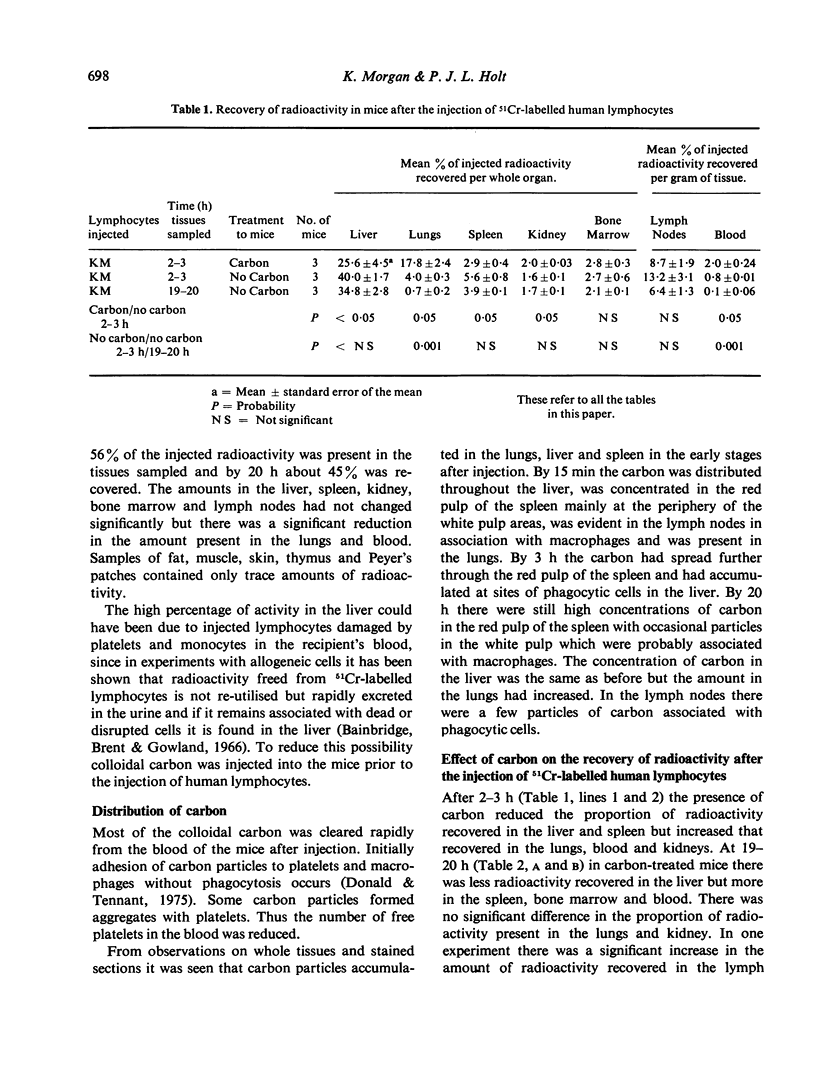
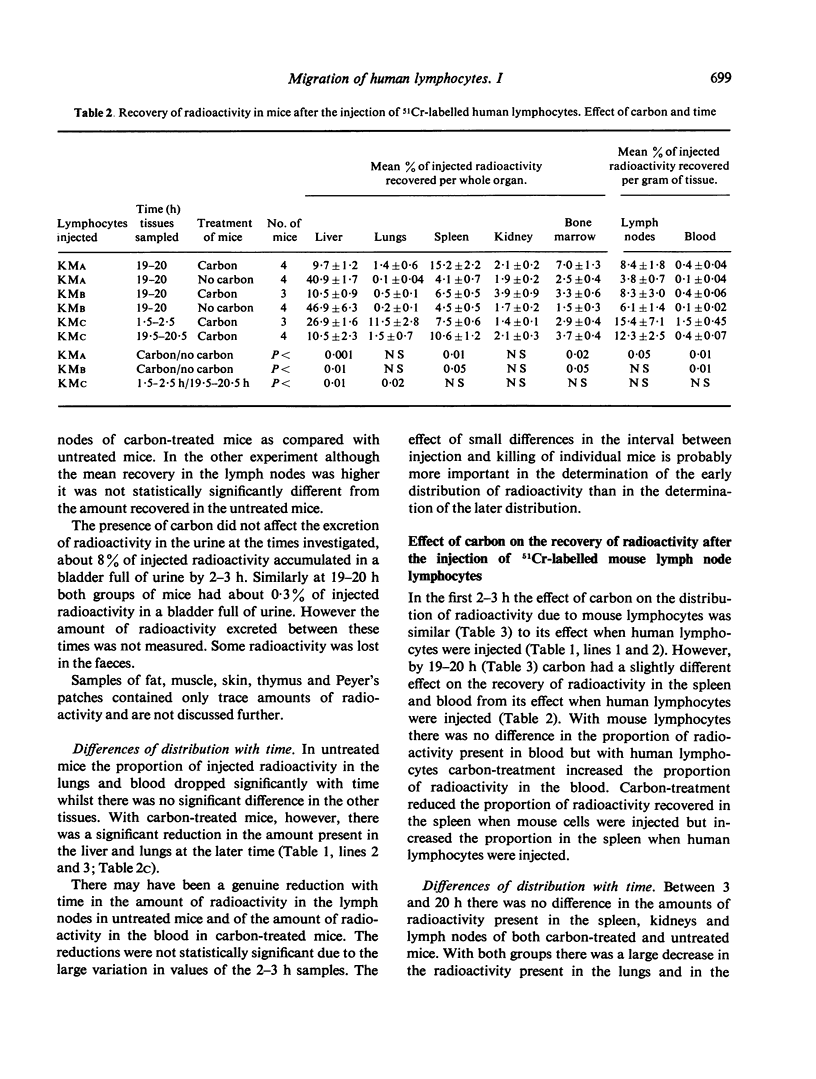
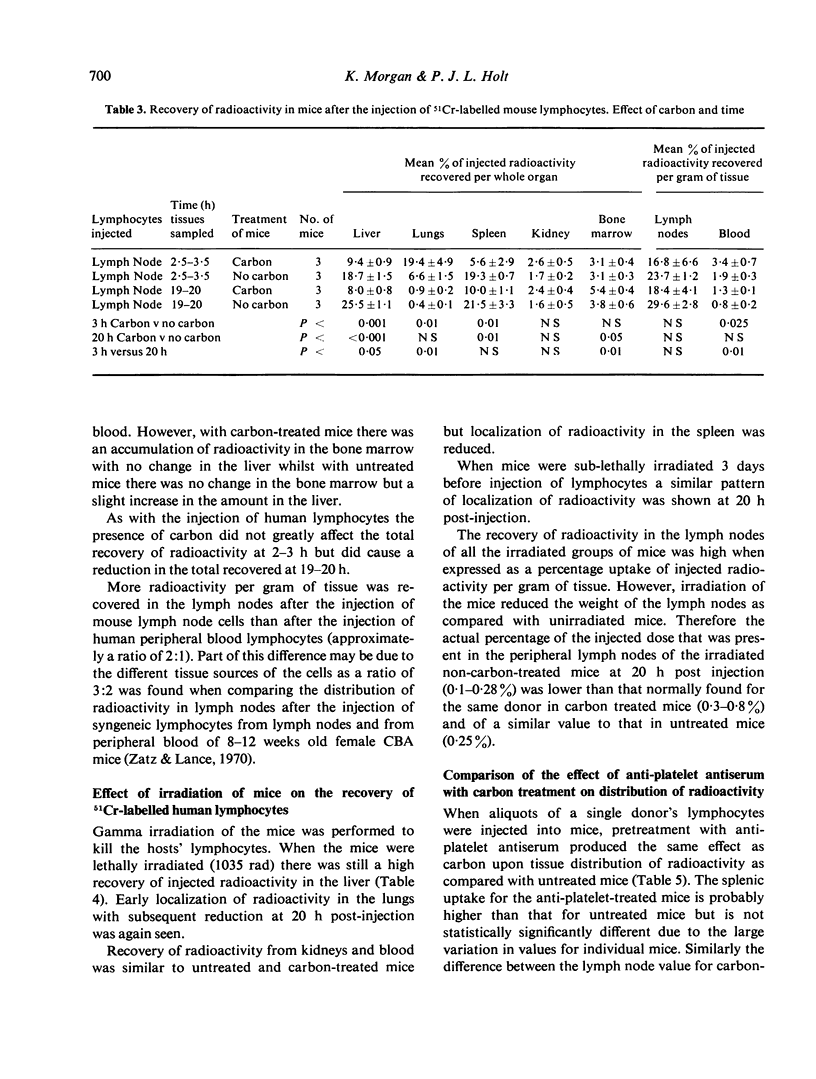
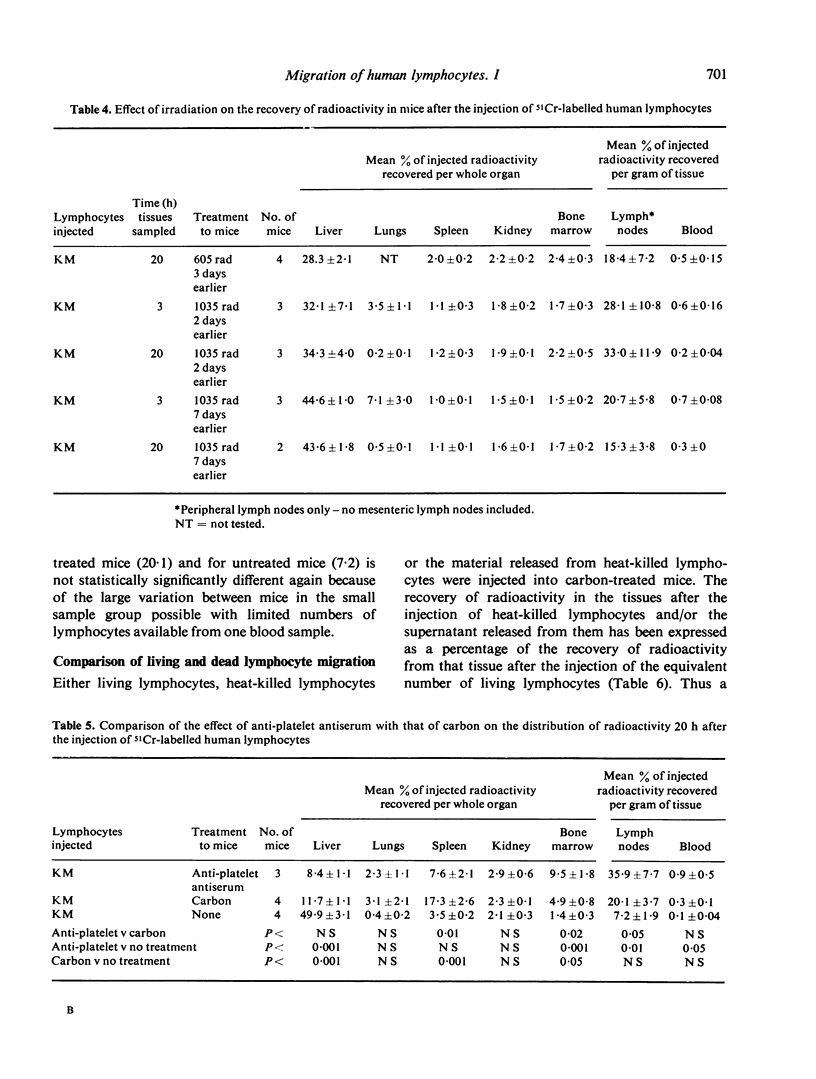
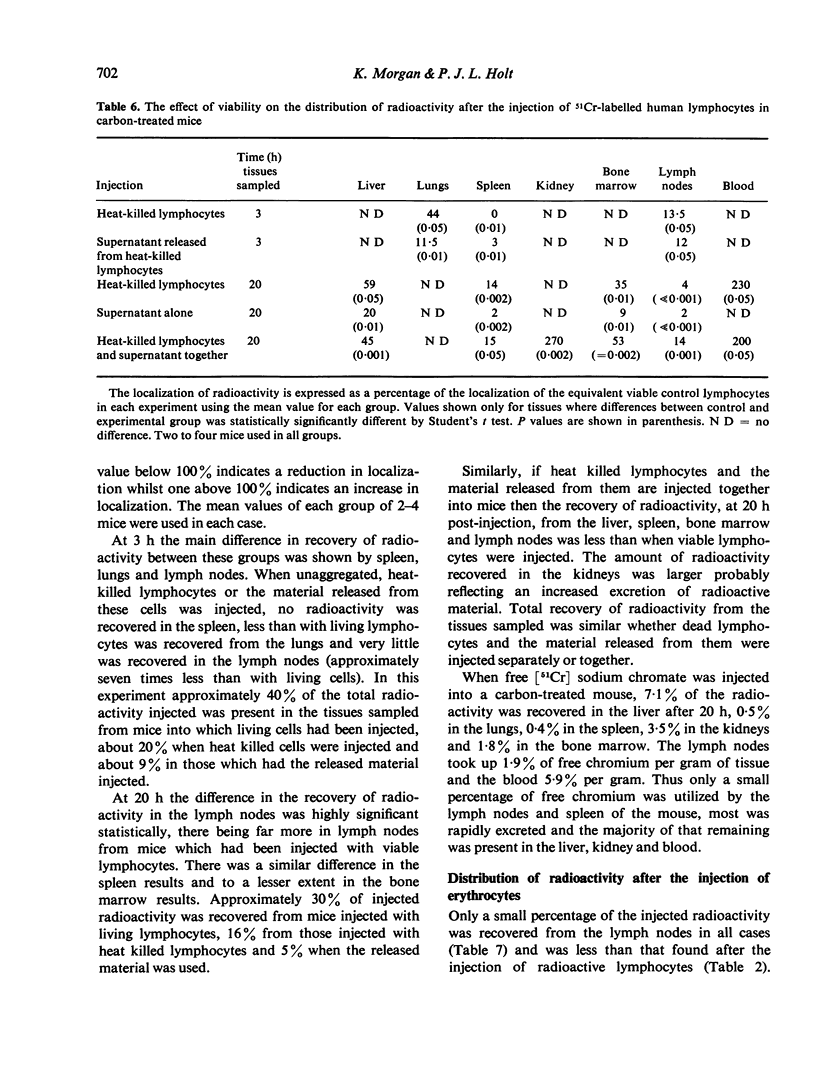
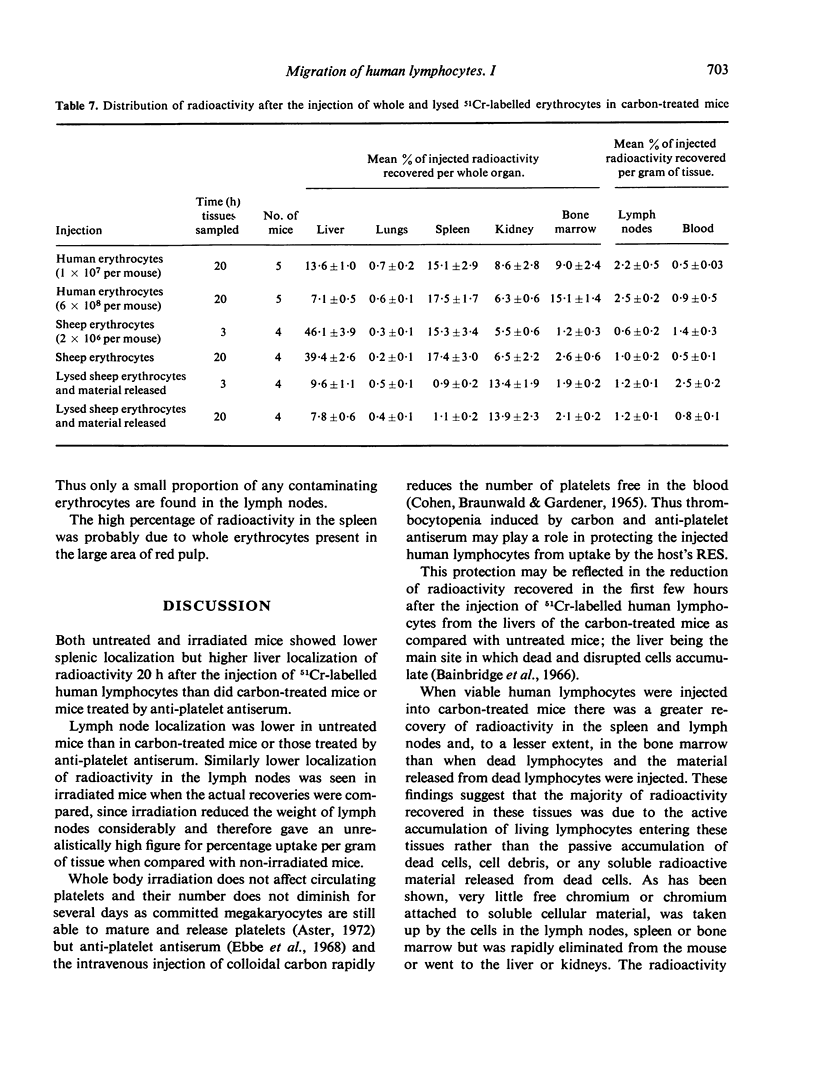
The distribution of radioactivity after the intravenous injection of 51Cr-labelled human lymphocytes has been examined in normal mice, irradiated mice, mice treated with anti-platelet antiserum and in mice treated with colloidal carbon. Pre-treatment with carbon and anti-platelet antiserum appears to protect the human lymphocytes from uptake by the host's reticuloendothelial system (RES). Comparison of tissue radioactivity in carbon-treated mice after the injection of viable human lymphocytes with that found after the injection of dead cells and soluble or insoluble cell debris showed that radioactivity recovered in the spleen and lymph nodes is primarily due to the migration of viable lymphocytes into these tissues. Thus the measurement of radioactivity in lymph nodes of carbon-treated mice after the injection of 51Cr-labelled human lymphocytes can be used as a model of these lymphocytes' ability to migrate into the lymph nodes during recirculation and to study factors influencing this migration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainbridge D. R., Brent L., Gowland G. Distribution of allogeneic 51Cr-labelled lymph node cells in mice. Transplantation. 1966 Mar;4(2):138–153. doi: 10.1097/00007890-196603000-00003. [DOI] [PubMed] [Google Scholar]
- COHEN P., BRAUNWALD J., GARDNER F. H. DESTRUCTION OF CANINE AND RABBIT PLATELETS FOLLOWING INTRAVENOUS ADMINISTRATION OF CARBON PARTICLES OR ENDOTOXIN. J Lab Clin Med. 1965 Aug;66:263–271. [PubMed] [Google Scholar]
- Donald K. J., Tennent R. J. The relative roles of platelets and macrophages in clearing particles from the blood; the value of carbon clearance as a measure of reticuloendothelial phagocytosis. J Pathol. 1975 Dec;117(4):235–245. doi: 10.1002/path.1711170406. [DOI] [PubMed] [Google Scholar]
- Ebbe S., Stohlman F., Jr, Overcash J., Donovan J., Howard D. Megakaryocyte size in thrombocytopenic and normal rats. Blood. 1968 Sep;32(3):383–392. [PubMed] [Google Scholar]
- Ford W. L., Atkins R. C. Specific unresponsiveness of recirculating lymphocytes ater exposure to histocompatibility antigen in F 1 hybrid rats. Nat New Biol. 1971 Dec 8;234(49):178–180. doi: 10.1038/newbio234178a0. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Gowans J. L. The traffic of lymphocytes. Semin Hematol. 1969 Jan;6(1):67–83. [PubMed] [Google Scholar]
- Frost P., Lance E. M. The cellular origin of the lymphochte trap. Immunology. 1974 Jan;26(1):175–186. [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959 Apr 23;146(1):54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans J. L. Life-span, recirculation, and transformation of lymphocytes. Int Rev Exp Pathol. 1966;5:1–24. [PubMed] [Google Scholar]
- HULSE E. V. QUANTITATIVE CELL COUNTS OF THE BONE MARROW AND BLOOD AND THEIR SECULAR VARIATIONS IN THE NORMAL ADULT RAT. Acta Haematol. 1964 Jan;31:50–63. doi: 10.1159/000209613. [DOI] [PubMed] [Google Scholar]
- Moore A. R., Hall J. G. Evidence for a primary association between immunoblasts and small gut. Nature. 1972 Sep 15;239(5368):161–162. doi: 10.1038/239161a0. [DOI] [PubMed] [Google Scholar]
- Ramasamy R. A fluorescent stain for viable rosette-forming cells. J Immunol Methods. 1974 Aug;5(3):305–306. doi: 10.1016/0022-1759(74)90116-1. [DOI] [PubMed] [Google Scholar]
- Souhami R. L. The effect of colloidal carbon on the organ distribution of sheep red cells and the immune response. Immunology. 1972 Apr;22(4):685–694. [PMC free article] [PubMed] [Google Scholar]
- Zatz M. M., Lance E. M. The distribution of 51Cr-labeled lymphocytes into antigen-stimulated mice. Lymphocyte trapping. J Exp Med. 1971 Jul 1;134(1):224–241. doi: 10.1084/jem.134.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M. M., Lance E. M. The distribution of chromium 51-labelled lymphoid cells in the mouse. A survey of anatomical compartments. Cell Immunol. 1970 May;1(1):3–17. doi: 10.1016/0008-8749(70)90057-2. [DOI] [PubMed] [Google Scholar]


