Abstract
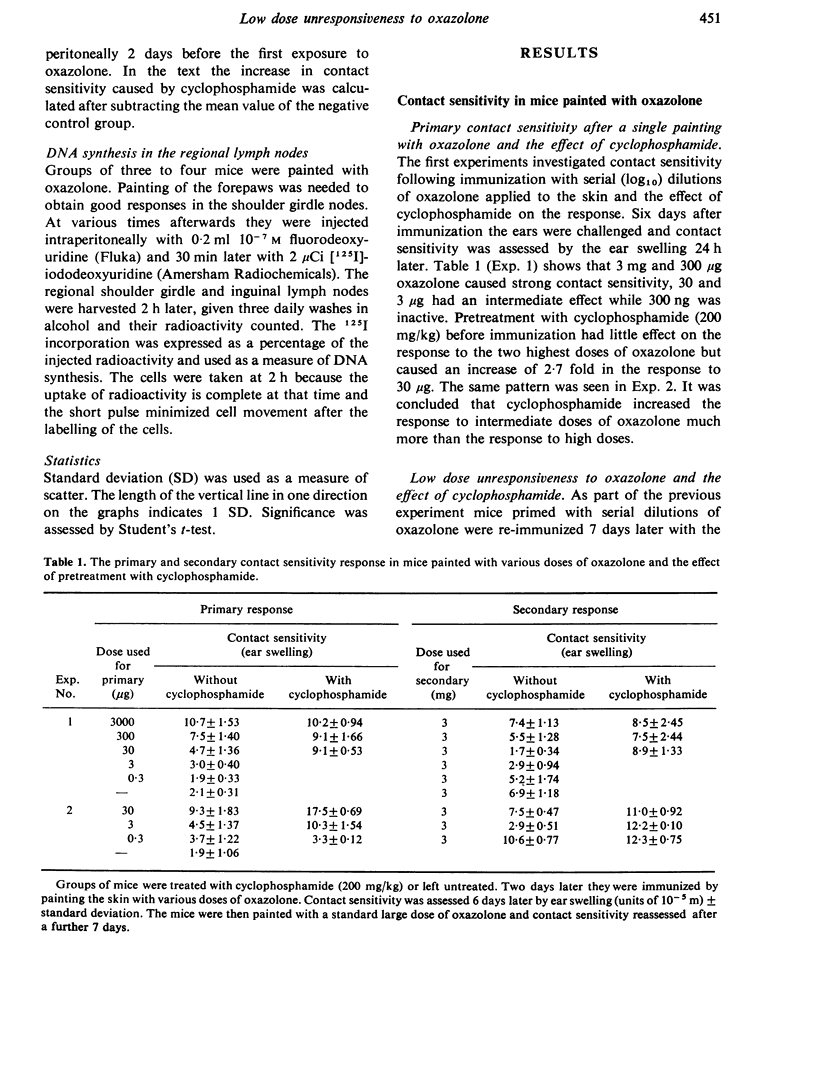
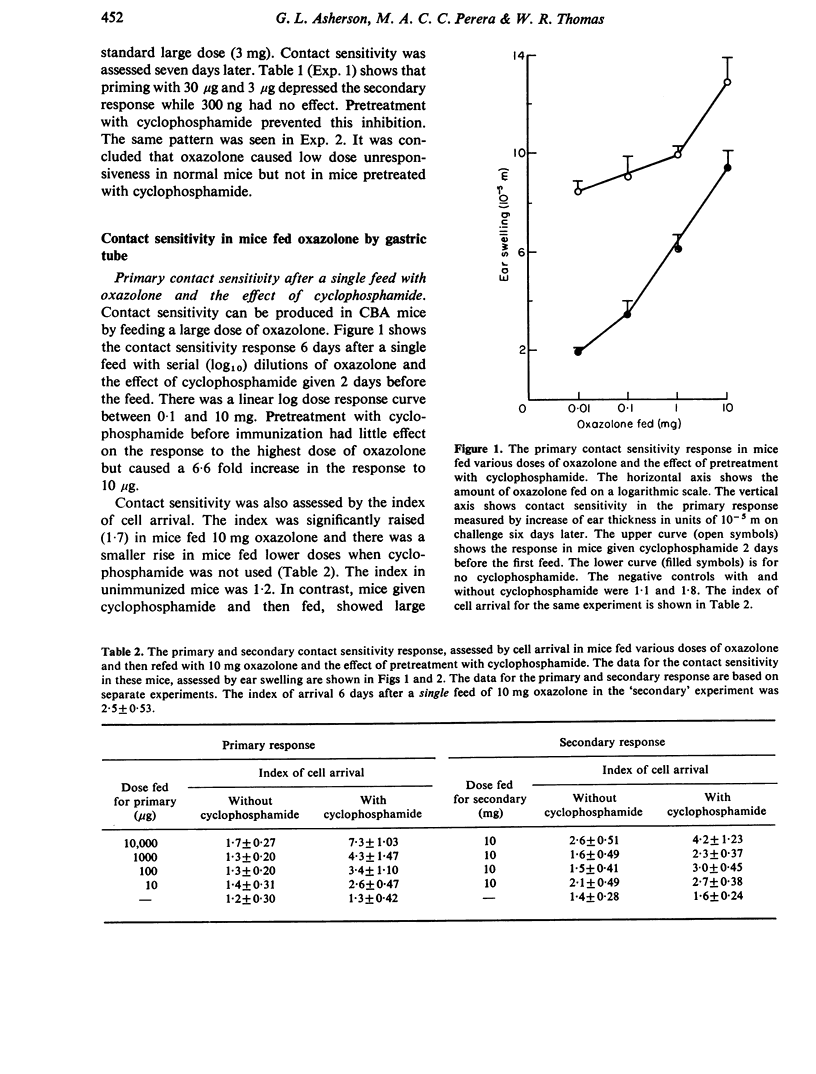
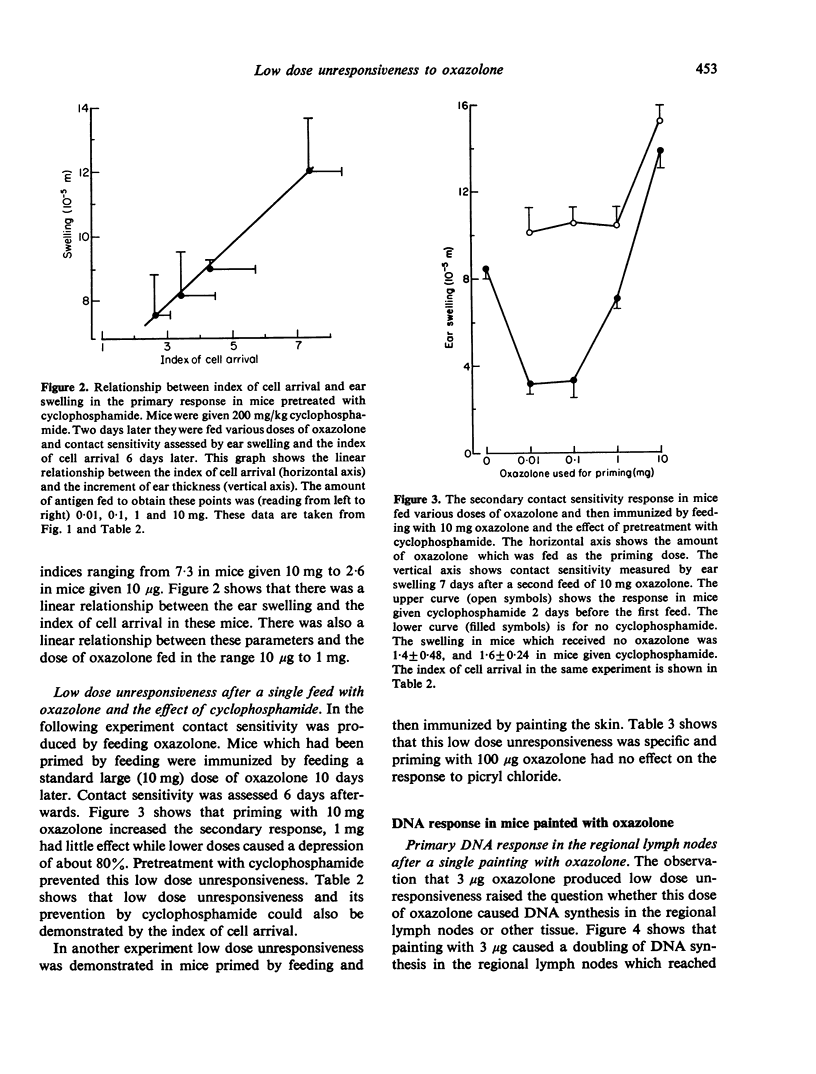
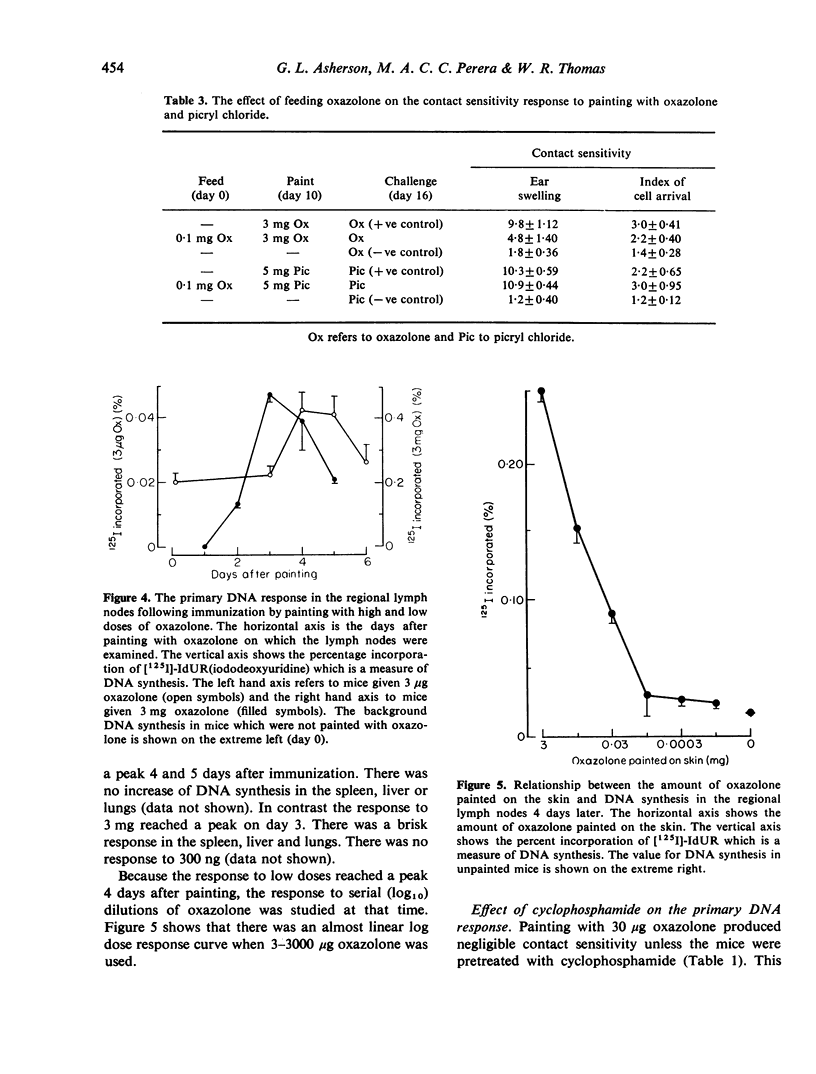
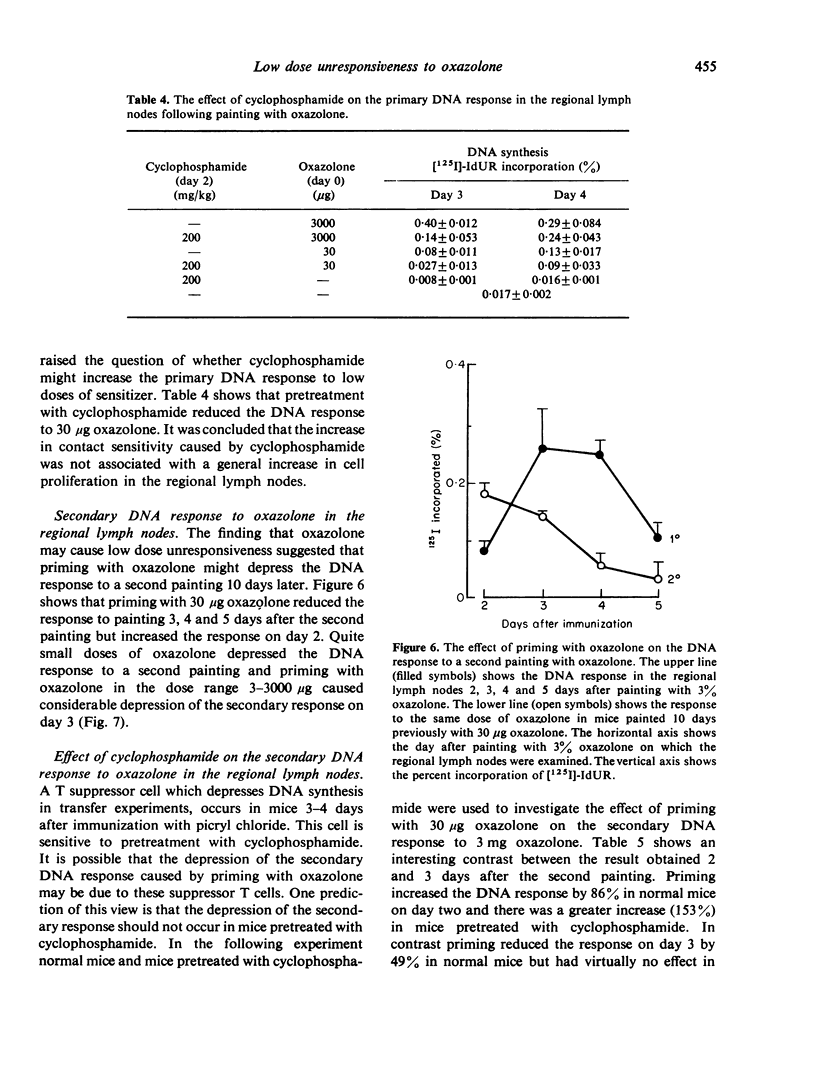
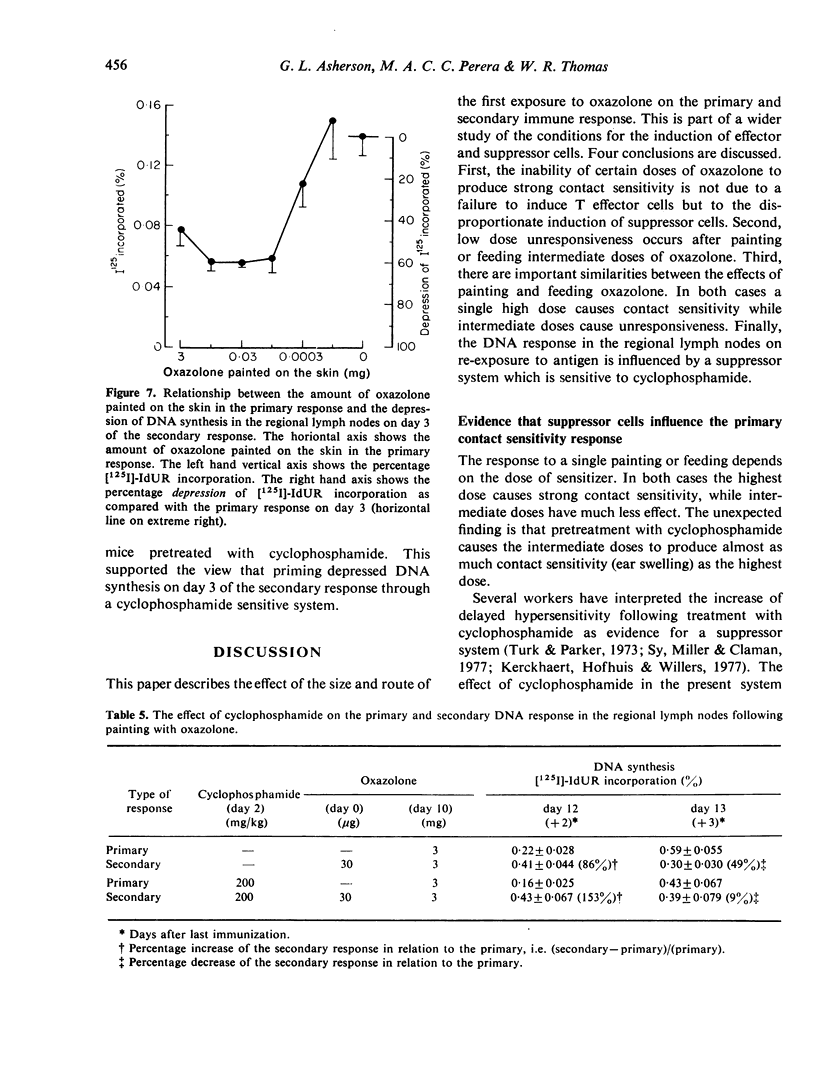
Cyclophosphamide was used to assess the role of suppressor cells in the contact sensitivity reaction. A single painting with 300 microgram and 30 microgram oxazolone produced poor contact sensitivity reactions (ear swelling). Cyclophosphamide (200 mg/kg) 2 days before painting increased the response to the lower doses but had less effect on the response to 3 mg oxazolone. A single feed with 10 mg oxazolone caused strong contact sensitivity while lower doses (10-1000 microgram) caused poor responses. Cyclophosphamide increased the response to the lower doses but not to the highest dose of oxazolone. These results suggested that the poor response to painting and feeding lower doses of oxazolone was due to a suppressor system which was sensitive to cyclophosphamide. A different result was obtained when contact sensitivity was measured by arrival of radioactively labelled cells. Cyclophosphamide had the greatest effect on cell arrival when high doses were fed. This indicates that ear swelling and cell arrival measure separate aspects of the contact sensitivity response. The lower doses of oxazolone, which caused little contact sensitivity, reduced the response to a standard immunizing dose. This low dose unresponsiveness occurred after either painting or feeding (Chase-Sulzberger phenomenon). It did not occur in mice treated with cyclophosphamide before the first exposure to oxazolone. This suggested that the low dose unresponsiveness was due to suppressor cells. The response to oxazolone was also assessed by DNA synthesis in the regional lymph nodes. A small dose of oxazolone (30 microgram) caused a peak of DNA synthesis on day four while a high dose (3 mg) caused a peak on day three. Pretreatment with cyclophosphamide depressed the response to 30 microgram although it increased contact sensitivity. The secondary response was smaller than the primary on days 3, 4 and 5 after immunization but larger on day two. The depression but not the increase was prevented by cyclophosphamide and was probably due to a suppressor system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Wood P. J., Mayhew B. Control of the immune response. I. Depression of DNA synthesis by immune lymph node cells. Immunology. 1975 Dec;29(6):1057–1065. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Mayhew B., Goldstein A. Adult thymectomy prevention of the appearance of suppressor T cells which depress contact sensitivity to picryl chloride and reversal of adult thymectomy effect by thymus extract. Eur J Immunol. 1976 Oct;6(10):699–703. doi: 10.1002/eji.1830061008. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Perera M. A., Mayhew B., Thomas W. R. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977 Sep;33(1):145–155. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Wood P. J. Proceedings: Control mechanisms in cell-mediated immunity. The separate control of net DNA synthesis and of contact sensitivity skin reactions and the role of thymus-derived cells. Monogr Allergy. 1974;8(0):154–167. [PubMed] [Google Scholar]
- Bonmassar E., Menconi E., Goldin A., Cudkowicz G. Escape of small numbers of allogeneic lymphoma cells from immune surveillance. J Natl Cancer Inst. 1974 Aug;53(2):475–479. doi: 10.1093/jnci/53.2.475. [DOI] [PubMed] [Google Scholar]
- Chiba J., Otokawa M., Egashira Y. Transient delayed-type hypersensitivity induced by lightly lipid-conjugated BSA and its conversion into sustained nature by cyclophosphamide treatment in guinea pigs. Jpn J Med Sci Biol. 1976 Oct;29(5):277–281. doi: 10.7883/yoken1952.29.277. [DOI] [PubMed] [Google Scholar]
- Colvin R. B., Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. II. Kinetics of fibrinogen/fibrin accumulation and vascular permeability changes in tuberculin and cutaneous basophil hypersensitivity reactions. J Immunol. 1975 Jan;114(1 Pt 2):377–387. [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. Effect of cyclophosphamide on delayed hypersensitivity to Staphylococcus aureus in mice. Immunology. 1977 Nov;33(5):767–776. [PMC free article] [PubMed] [Google Scholar]
- Kerckhaert J. A., Hofhuis F. M., Willers J. M. Influence of cyclophosphamide on delayed hypersensitivity and acquired cellular resistance to Listeria monocytogenes in the mouse. Immunology. 1977 Jun;32(6):1027–1032. [PMC free article] [PubMed] [Google Scholar]
- Kilshaw P. J., Brent L., Thomas A. V. Specific unresponsiveness to skin allografts in mice. II. The mechanism of unresponsiveness induced by tissue extracts and antilymphocytic serum. Transplantation. 1974 Jan 1;17(1):57–69. [PubMed] [Google Scholar]
- Lowney E. D. Immunologic unresponsiveness appearing after topical application of contact sensitizers to the guinea pig. J Immunol. 1965 Sep;95(3):397–403. [PubMed] [Google Scholar]
- Macher E., Chase M. W. Studies on the sensitization of animals with simple chemical compounds. XII. The influence of excision of allergenic depots on onset of delayed hypersensitivity and tolerance. J Exp Med. 1969 Jan 1;129(1):103–121. doi: 10.1084/jem.129.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Noble B., Parker D., Scheper R. J., Turk J. L. The relation between B-cell stimulation and delayed hypersensitivity. The effect of cyclophosphamide pretreatment on antibody production. Immunology. 1977 Jun;32(6):885–891. [PMC free article] [PubMed] [Google Scholar]
- Scheper R. J., Noble B., Parker D., Turk J. L. The value of an assessment of erythema and increase in thickness of the skin reaction for a full appreciation of the nature of delayed hypersensitivity in the guinea pig. Int Arch Allergy Appl Immunol. 1977;54(1):58–66. doi: 10.1159/000231808. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Parker D., Noble B., Turk J. L. The relation of immune depression and B-cell stimulation during the development of delayed hypersensitivity to soluble antigens. Immunology. 1977 Feb;32(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Claman H. N. Immune suppression with supraoptimal doses of antigen in contact sensitivity. I. Demonstration of suppressor cells and their sensitivity to cyclophosphamide. J Immunol. 1977 Jul;119(1):240–244. [PubMed] [Google Scholar]
- Takahashi C., Nishikawa S., Katsura Y., Izumi T. Anti-DNP antibody response after the topical application of DNFB in mice. Immunology. 1977 Oct;33(4):589–596. [PMC free article] [PubMed] [Google Scholar]
- Thomas W. R., Asherson G. L., Watkins M. C. Reaginic antibody produced in mice with contact sensitivity. J Exp Med. 1976 Nov 2;144(5):1386–1390. doi: 10.1084/jem.144.5.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D. Further studies on B-lymphocyte suppression in delayed hypersensitivity, indicating a possible mechanism for Jones-Mote hypersensitivity. Immunology. 1973 Apr;24(4):751–758. [PMC free article] [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., Gamble J., Whitelaw A. A radioisotopic method to measure delayed type hypersensitivity in the mouse. I. Studies in sensitized and normal mice. Int Arch Allergy Appl Immunol. 1975;49(5):670–692. doi: 10.1159/000231449. [DOI] [PubMed] [Google Scholar]
- Wood P., Asherson G. L., Mayhew B., Thomas W. R., Zembala M. Control of the immune reaction: T cells in immunized mice which depress the in vivo DNA synthesis response in the lymph nodes to skin painting with the contact sensitizing agent picryl chloride. Cell Immunol. 1977 Apr;30(1):25–34. doi: 10.1016/0008-8749(77)90044-2. [DOI] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Noworolski J., Mayhew B. Contact sensitivity to picryl chloride: the occurrence of B suppressor cells in the lymph nodes and spleen of immunized mice. Cell Immunol. 1976 Aug;25(2):266–278. doi: 10.1016/0008-8749(76)90117-9. [DOI] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. The effect of cyclophosphamide and irradiation on cells which suppress contact sensitivity in the mouse. Clin Exp Immunol. 1976 Mar;23(3):554–561. [PMC free article] [PubMed] [Google Scholar]


