Abstract
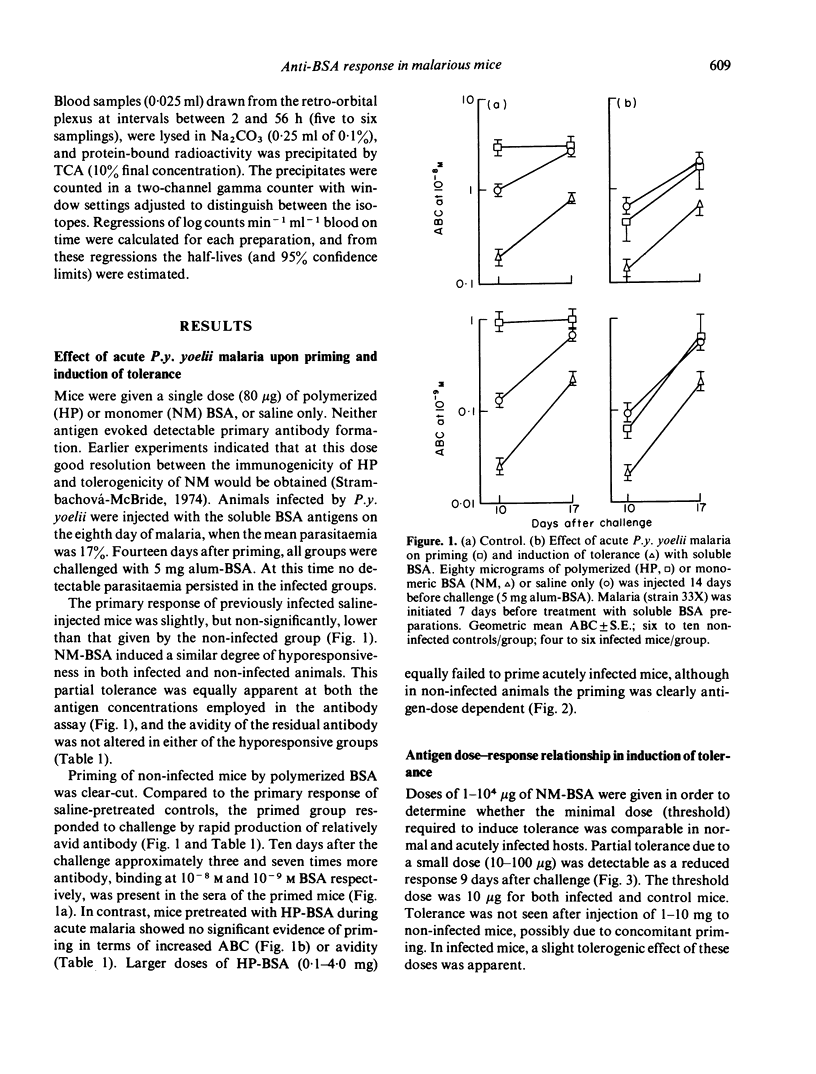
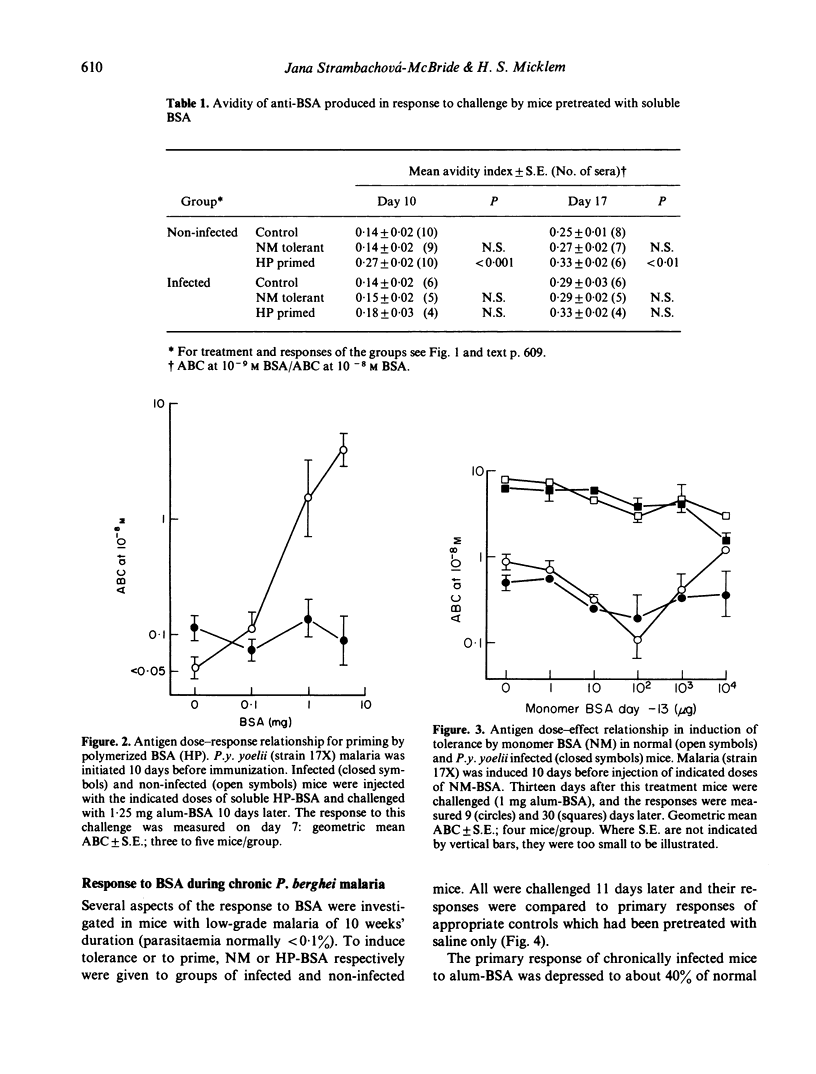
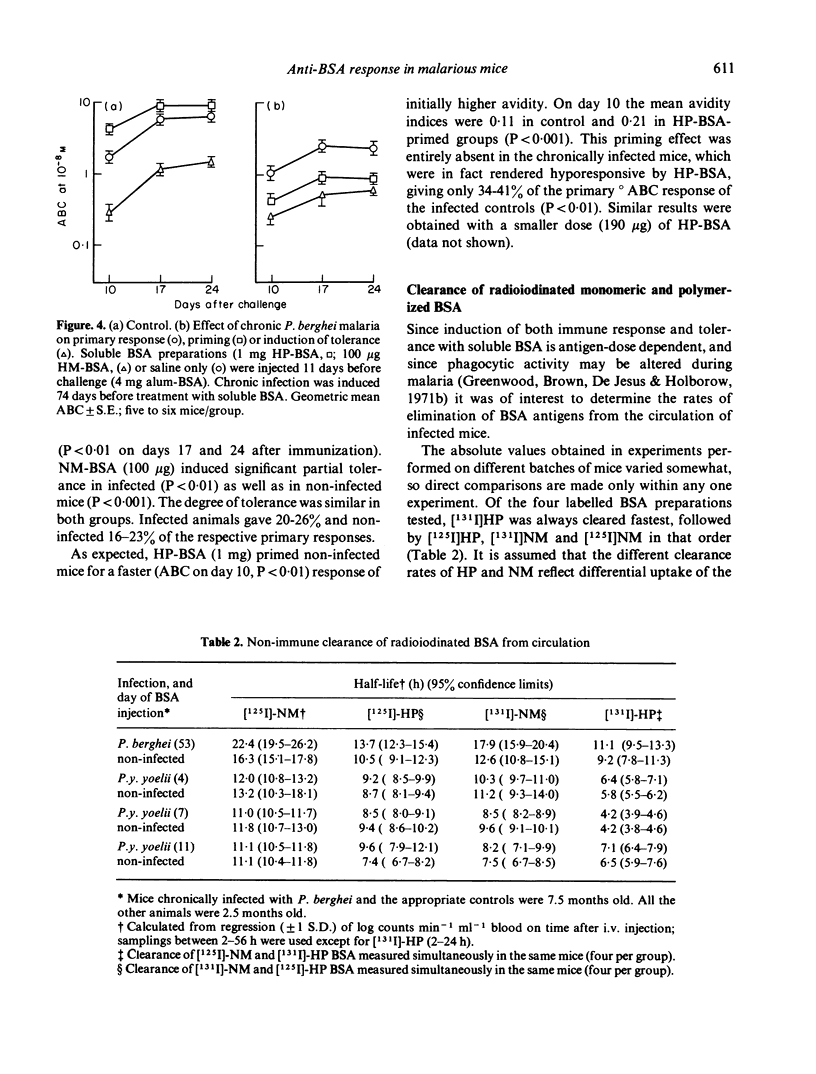
The primary antibody response to alumadsorbed BSA was depressed when initiated during low-grade chronic Plasmodium berghei malaria in mice, as previously reported during acute P.y. yoelii infection. Induction of immunological memory by soluble polymerized BSA was abolished in both infections; in infected hosts this normally immunogenic stimulus resulted in partial tolerance. In contrast to the depression of immune response, neither infection interfered with the induction of low-zone tolerance by monomeric BSA. The rate of non-immune elimination of BSA was found to be normal during acute malaria, and only slightly reduced in chronic infection. These results may be explained in terms of abnormal antigen handling in infected mice, due to some functional defect in macrophages, although this does not seem to be a sufficient explanation for all the phenomena of malaria-associated immunosuppression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown I. N., Watson S. R., Sljivić V. S. Antibody response in vitro of spleen cells from Plasmodium yoelii-infected mice. Infect Immun. 1977 May;16(2):456–460. doi: 10.1128/iai.16.2.456-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell W., Elko E. E. Effect of splenectomy on phagocytic activation by Plasmodium berghei. J Infect Dis. 1966 Oct;116(4):429–438. doi: 10.1093/infdis/116.4.429. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. Tolerance induction as a model for cell differentiation. Br Med Bull. 1976 May;32(2):147–151. doi: 10.1093/oxfordjournals.bmb.a071347. [DOI] [PubMed] [Google Scholar]
- FREI P. C., BENACERRAF B., THORBECKE G. J. PHAGOCYTOSIS OF THE ANTIGEN, A CRUCIAL STEP IN THE INDUCTION OF THE PRIMARY RESPONSE. Proc Natl Acad Sci U S A. 1965 Jan;53:20–23. doi: 10.1073/pnas.53.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei P. C., Cruchaud S., Arnaudo L., Vannotti A. Immunologic tolerance induced in the adult rabbit with modified BSA. J Immunol. 1968 Sep;101(3):605–610. [PubMed] [Google Scholar]
- Goodwin L. G., Green D. G., Guy M. W., Voller A. Immunosuppression during trypanosomiasis. Br J Exp Pathol. 1972 Feb;53(1):40–43. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Brown J. C., De Jesus D. G., Holborow E. J. Immunosuppression in murine malaria. II. The effect on reticulo-endothelial and germinal centre function. Clin Exp Immunol. 1971 Sep;9(3):345–354. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Kitchen A. G., Di Luzio N. R. Influence of Plasmodium berghei infections on phagocytic and humoral recognition factor activity. J Reticuloendothel Soc. 1971 Mar;9(3):237–247. [PubMed] [Google Scholar]
- Kölsch E., Mitchison N. A. The subcellular distribution of antigen in macrophages. J Exp Med. 1968 Nov 1;128(5):1059–1079. doi: 10.1084/jem.128.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölsch E., Stumpf R., Weber G. Low zone tolerance and suppressor T cells. Transplant Rev. 1975;26:56–86. doi: 10.1111/j.1600-065x.1975.tb00175.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. C., Shiozawa C., Shaw A., Diener E. Requirement for accessory cells in the antibody response to T cell-independent antigens in vitro. Eur J Immunol. 1976 Jan;6(1):63–68. doi: 10.1002/eji.1830060114. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Micklem H. S. Immunosuppression in murine malaria. II. The primary response to bovine serum albumin. Immunology. 1977 Aug;33(2):253–259. [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Micklem H. S., Ure J. M. Immunosuppression in murine malaria. I. Response to type III pneumococcal polysaccharide. Immunology. 1977 May;32(5):635–644. [PMC free article] [PubMed] [Google Scholar]
- Orsin F., Shulman S. The antigens and autoantigens of the seminal vesicle. I. Immunochemical studies on guinea pig vesicular fluid. J Exp Med. 1971 Jul 1;134(1):120–140. doi: 10.1084/jem.134.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckard R. N., Weir D. M., McBride W. H. Factors influencing the immune response. 3. The blocking effect of Corynebacterium parvum upon the induction of acquired immunological unresponsiveness to bovine serum albumin in the adult rabbit. Clin Exp Immunol. 1968 Jun;3(5):413–421. [PMC free article] [PubMed] [Google Scholar]
- Poels L. G., van Niekerk C. C. Plasmodium berghei: immunosuppression and hyperimmunoglobulinemia. Exp Parasitol. 1977 Jun;42(1):235–247. doi: 10.1016/0014-4894(77)90081-9. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Brenig C. Paralysis to serum albumins in T and B lymphocytes in mice. Dose dependence, specificity and kinetics of escape. Eur J Immunol. 1974 Feb;4(2):120–125. doi: 10.1002/eji.1830040211. [DOI] [PubMed] [Google Scholar]
- Salaman M. H., Wedderburn N., Bruce-Chwatt L. J. The immunodepressive effect of a murine plasmodium and its interaction with murine oncogenic viruses. J Gen Microbiol. 1969 Dec;59(3):383–391. doi: 10.1099/00221287-59-3-383. [DOI] [PubMed] [Google Scholar]
- Schmidtke J. R., Unanue E. R. Macrophage-antigen interaction: uptake, metabolism and immunogenicity of foreign albumin. J Immunol. 1971 Aug;107(2):331–338. [PubMed] [Google Scholar]
- Spira D. T., Golenser J., Gery I. The reactivity of spleen cells from malarious rats to non-specific mitogens. Clin Exp Immunol. 1976 Apr;24(1):139–145. [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Allison A. C. Mode of action of adjuvants: effects on antibody responses to macrophage-associated bovine serum albumin. J Immunol. 1970 Jan;104(1):128–139. [PubMed] [Google Scholar]
- Takemori T., Tada T. Selective roles of thymus-derived lymphocytes in the antibody response. II. Preferential suppression of high-affinity antibody-forming cells by carrier-primed suppressor T cells. J Exp Med. 1974 Jul 1;140(1):253–266. doi: 10.1084/jem.140.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Waki S., Takada S., Suzuki M. Plasmodium berghei: suppressed response of antibody-forming cells in infected mice. Exp Parasitol. 1977 Oct;43(1):143–152. doi: 10.1016/0014-4894(77)90017-0. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- Wedderburn N., Dracott B. N. The immune reponse to type III pneumococcal polysaccharide in mice with malaria. Clin Exp Immunol. 1977 Apr;28(1):130–137. [PMC free article] [PubMed] [Google Scholar]
- Weigle W. O., High G. J. The effect of antigenic competition on antibody production to heterologous proteins, termination of immunologic unresponsiveness and induction of autoimmunity. J Immunol. 1967 Aug;99(2):392–398. [PubMed] [Google Scholar]
- Zan-Bar I., Nachtigal D., Feldman M. Mechanisms in immune tolerance. II. Specific immunosuppression by T lymphocytes of B memory cells in mice made tolerant to HSA. Cell Immunol. 1975 May;17(1):202–214. doi: 10.1016/s0008-8749(75)80020-7. [DOI] [PubMed] [Google Scholar]
- Zitron I. M., Mosier D. E., Paul W. E. The role of surface IgD in the response to thymic-independent antigens. J Exp Med. 1977 Dec 1;146(6):1707–1718. doi: 10.1084/jem.146.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]



