Abstract
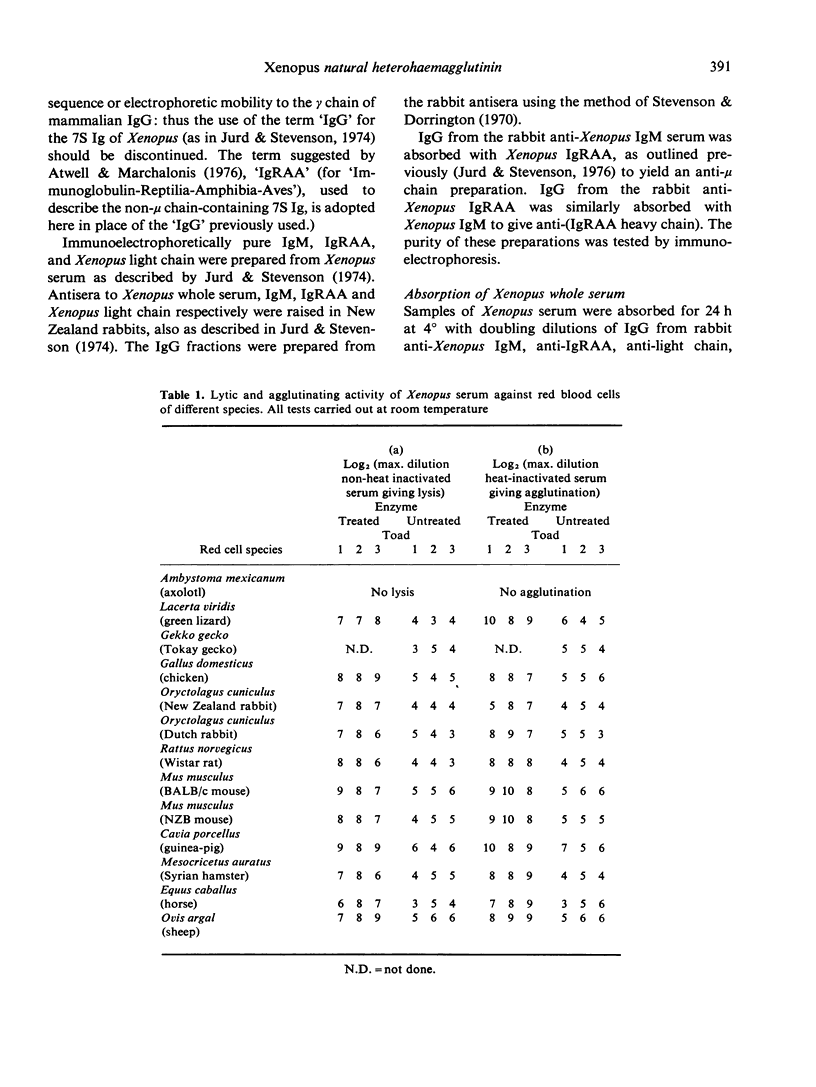
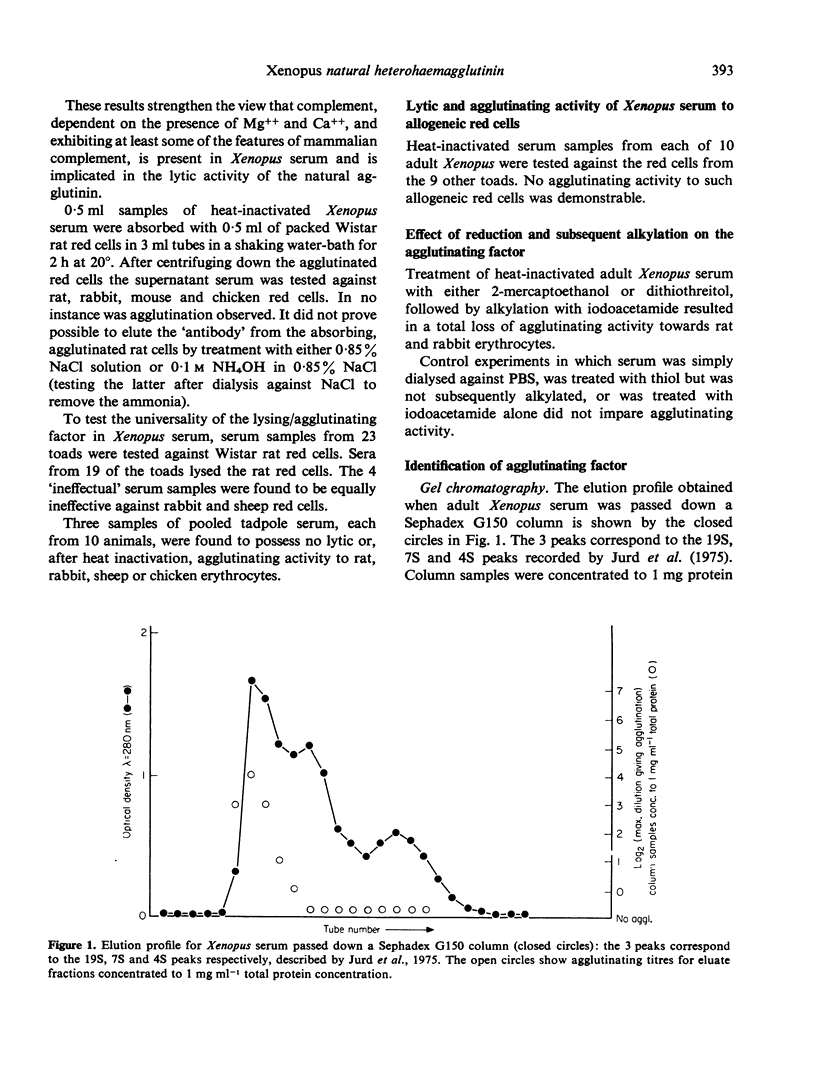
In most adult Xenopus laevis the serum contains a 'natural' factor capable of lysing the erythrocytes from a wide variety of amniote species. The factor has no effect on the erythrocytes of another amphibian, Ambystoma mexicanum, nor will serum from one animal lyse red cells from another Xenopus individual. No lysing factor was present in the serum of larval (tadpole) Xenopus. Heating of Xenopus serum to 56 degrees for 30 min, absorption of the serum with zymosan or inulin, or removal of calcium and magnesium ions results in loss of lytic activity, although haemagglutinating activity remains, suggesting that the factor can fix complement. The factor elutes from a gel chromatography column in the 19S peak, and is inactivated by thiol reduction and subsequent alkylation. These findings, coupled with immunoabsorption studies suggest that the haemagglutinin is an immunoglobulin of the IgM class. The significance of this suggestion is discussed in the light of previous reports of 'natural' heterohaemagglutinins in other species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balding P., Gold E. R. The natural heterohaemagglutinin in the serum of the toad Bufo regularis, and its relationship to lower vertebrate immunoglobulins. Immunology. 1976 May;30(5):769–777. [PMC free article] [PubMed] [Google Scholar]
- Bezkorovainy A., Springer G. F., Desai P. R. Physicochemical properties of the eel anti-human blood-group H(O) antibody. Biochemistry. 1971 Sep 28;10(20):3761–3764. doi: 10.1021/bi00796a018. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Hodgins H. O., Weiser R. S. Antibody response in rainbow trout (Salmo gairdneri) II. Studies on the kinetics of development of antibody-producing cells and on complement and natural hemolysin. J Immunol. 1969 May;102(5):1202–1207. [PubMed] [Google Scholar]
- Clem I. W., De Boutaud F., Sigel M. M. Phylogeny of immunoglobulin structure and function. II. Immunoglobulins of the nurse shark. J Immunol. 1967 Dec;99(6):1226–1235. [PubMed] [Google Scholar]
- Frair W. Blood Group Studies with Turtles. Science. 1963 Jun 28;140(3574):1412–1414. doi: 10.1126/science.140.3574.1412-a. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Balding P. Short communication structure of 'natural antibodies' in lower vertebrates. J Immunogenet. 1976 Jun;3(3):207–209. doi: 10.1111/j.1744-313x.1976.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Hadji-Azimi I. Studies on Xenopus laevis immunoglobulins. Immunology. 1971 Sep;21(3):463–473. [PMC free article] [PubMed] [Google Scholar]
- Harisdangkul V., Kabat E. A., McDonough R. J., Sigel M. M. A protein in normal nurse shark serum which reacts specifically with fructosans. I. Purification and immunochemical characterization. J Immunol. 1972 May;108(5):1244–1258. [PubMed] [Google Scholar]
- Harisdangkul V., Kabat E. A., McDonough R. J., Sigel M. M. A protein in normal nurse shark serum which reacts specifically with fructosans. II. Physicochemical studies. J Immunol. 1972 May;108(5):1259–1270. [PubMed] [Google Scholar]
- Jurd R. D., Luther-Davies S. M., Stevenson G. T. Humoral antibodies to soluble antigens in larvae of Xenopus laevis. Comp Biochem Physiol B. 1975 Jan 15;50(1):65–70. doi: 10.1016/0305-0491(75)90300-4. [DOI] [PubMed] [Google Scholar]
- Jurd R. D., Stevenson G. T. Surface immunoglobulins on Xenopus laevis lymphocytes. Comp Biochem Physiol A Comp Physiol. 1976;53(4):381–387. doi: 10.1016/s0300-9629(76)80160-0. [DOI] [PubMed] [Google Scholar]
- Potter M. Myeloma proteins (M-components) with antibody-like activity. N Engl J Med. 1971 Apr 15;284(15):831–838. doi: 10.1056/NEJM197104152841507. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Voss E. W., Sigel M. M. Biological and chemical properties of natural antibodies in the nurse shark. J Immunol. 1970 Dec;105(6):1344–1352. [PubMed] [Google Scholar]
- Stevenson G. T., Dorrington K. J. The recombination of dimers of immunoglobulin peptide chains. Biochem J. 1970 Aug;118(5):703–712. doi: 10.1042/bj1180703. [DOI] [PMC free article] [PubMed] [Google Scholar]


