Abstract
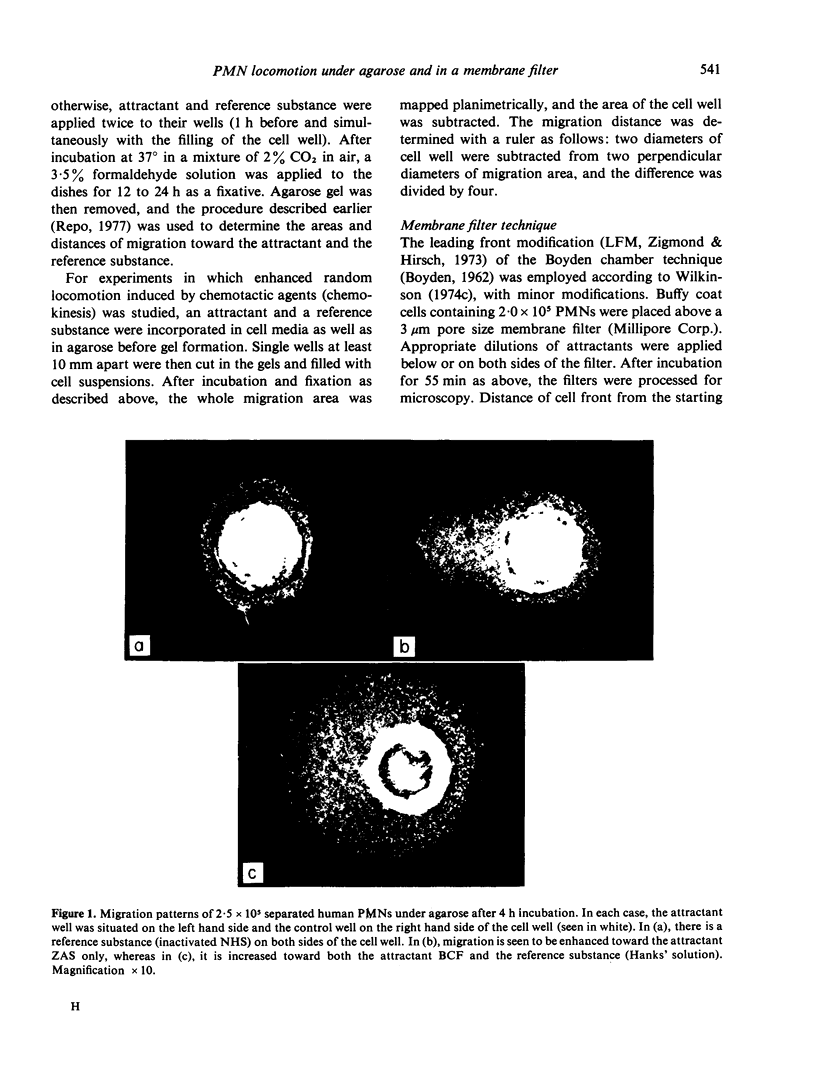
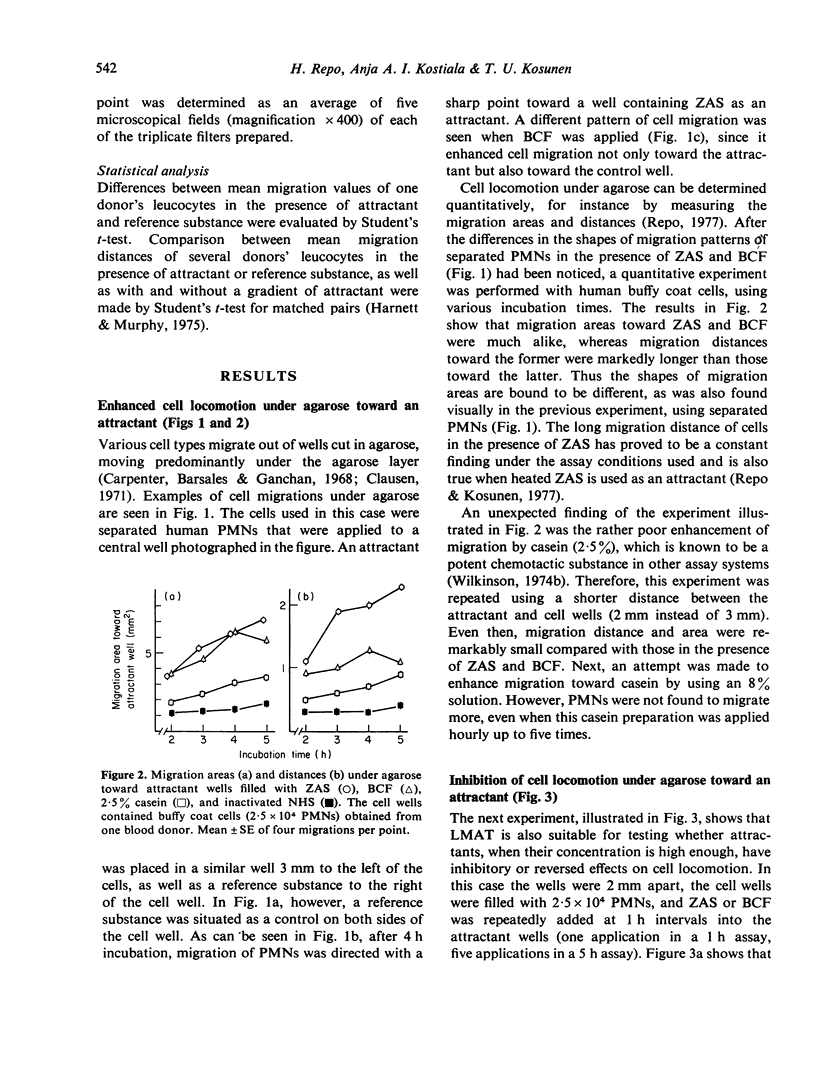
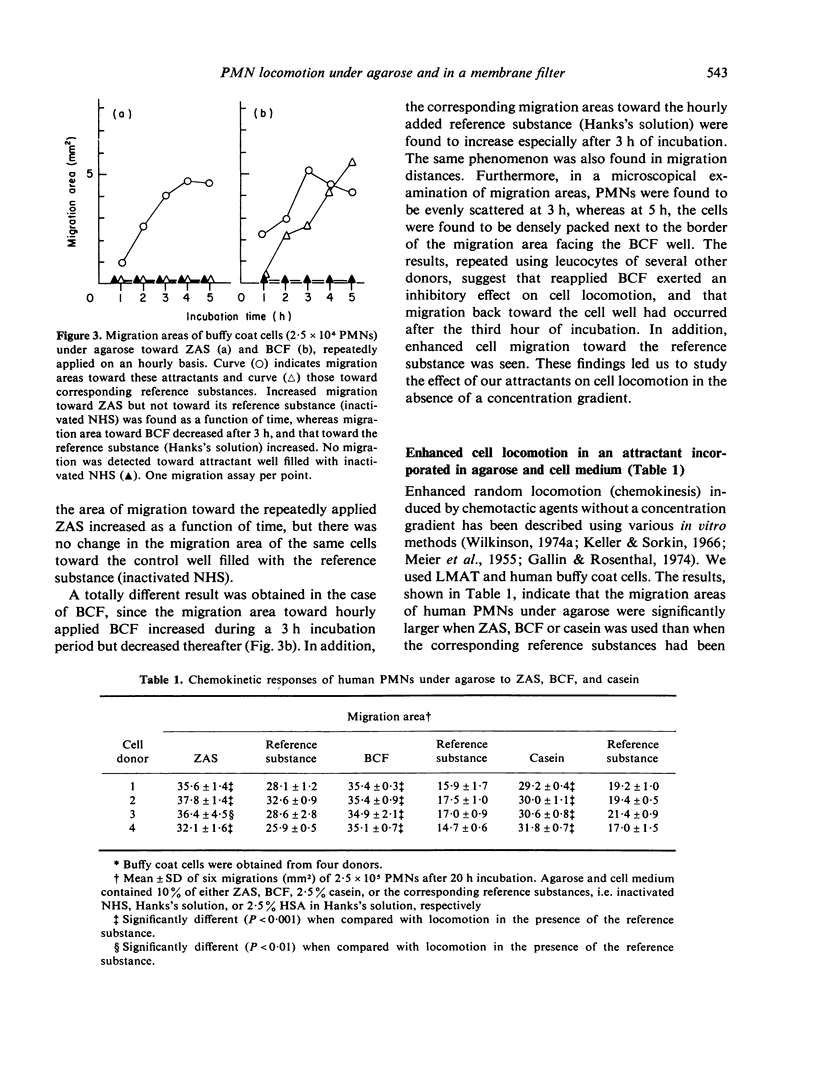
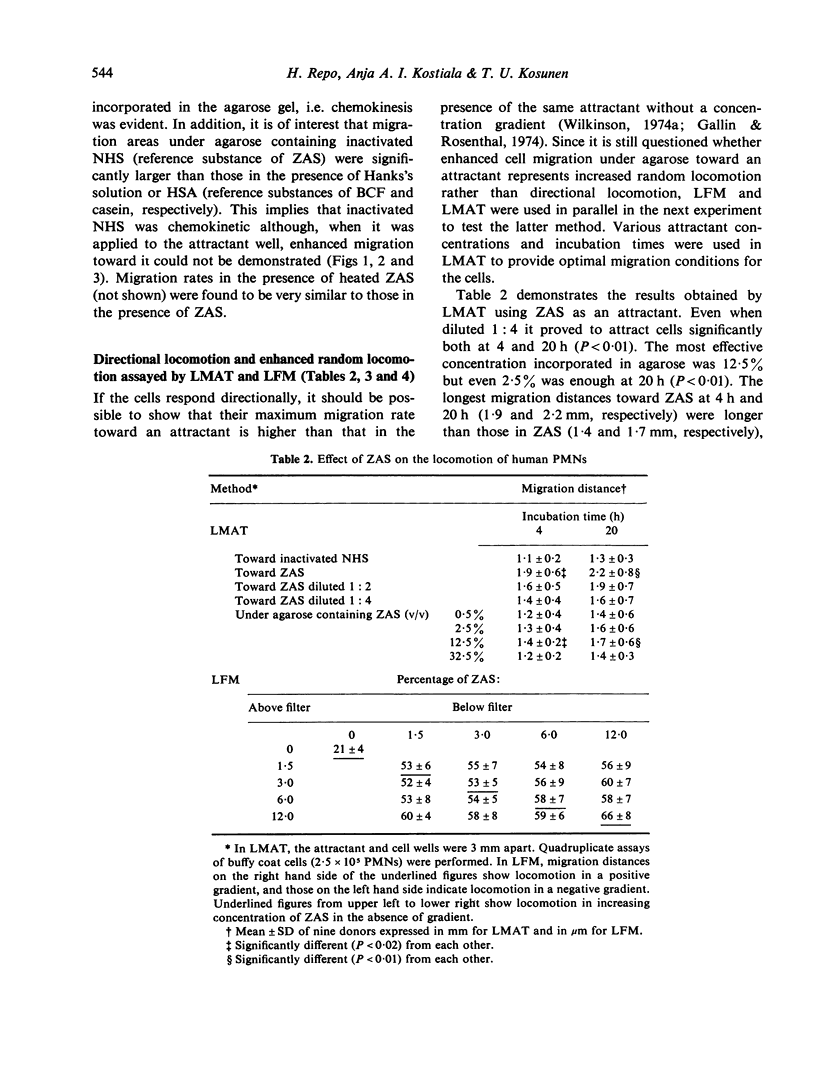
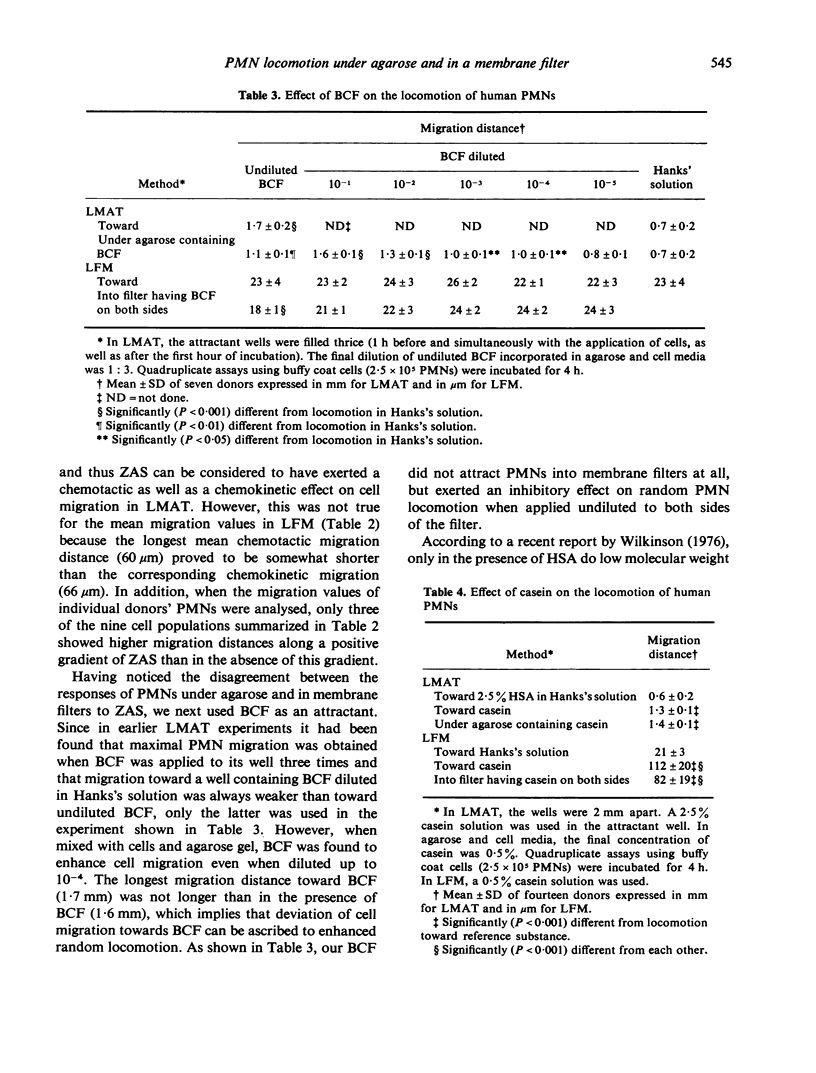
Three different cell attractants, together with the parallel use of the leucocyte migration agarose test (LMAT) and the leading front modification (LFM) of the Boyden chamber technique, were employed in studying whether the maximal migration of normal human polymorphonuclear leucocytes (PMNs) is higher toward an attractant (chemotaxis) than in the same attractant incorporated in the culture media (chemokinesis). Using LMAT, the maximal migration distance toward zymosan activated serum (ZAS) was found to be significantly longer than that under agarose mixed with ZAS, thus indicating a chemotactic effect exerted by ZAS. When bacterial culture filtrate (BCF) and casein were used as attractants, the corresponding difference was not significant, implying that the stimulatory effect of these substances on cell migration could be explained by increased random locomotion (chemokinesis) alone. In LFM, the migration rate was significantly higher along a casein gradient than without a gradient. Using ZAS, however, only chemokinesis could be demonstrated. BCF was found to attract PMNs into membrane filters only in the presence of human serum albumin. These observations give credence to the view that both LMAT and LFM are applicable to the in vitro assessment of chemotaxis and chemokinesis but the attractant of choice for this is different in each of the two methods.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Carpenter R. R., Barsales P. B., Ganchan R. P. Antigen-induced inhibition of cell migration in agar gel, plasma clot, and liquid media. J Reticuloendothel Soc. 1968 Oct;5(5):472–483. [PubMed] [Google Scholar]
- Cutler J. E., Munoz J. J. A simple in vitro method for studies on chemotaxis. Proc Soc Exp Biol Med. 1974 Nov;147(2):471–474. doi: 10.3181/00379727-147-38367. [DOI] [PubMed] [Google Scholar]
- Frei P. C., Baisero M. H., Ochsner M. Chemotaxis of human polymorphonuclears in vitro. II. Technical study. J Immunol Methods. 1974 Oct;5(4):375–386. doi: 10.1016/0022-1759(74)90021-0. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Biggar W. D. Influence of serum-derived chemotactic factors and bacterial products on human neutrophil chemotaxis. Infect Immun. 1977 Jan;15(1):212–220. doi: 10.1128/iai.15.1.212-220.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T. J., Sieber O. F., Jr Chemotactic migration of neutrophils under agarose. Life Sci. 1976 Jan 15;18(2):177–181. doi: 10.1016/0024-3205(76)90022-9. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Borel J. F., Wilkinson P. C., Hess M. W., Cottier H. Re-assessment of Boyden's technique for measuring chemotaxis. J Immunol Methods. 1972 Jan;1(2):165–168. doi: 10.1016/0022-1759(72)90043-9. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Wilkinson P. C., Abercrombie M., Becker E. L., Hirsch J. G., Miller M. E., Scottramsey W., Zigmond S. H. A proposal for the definition of terms related to locomotion of leucocytes and other cells. Clin Exp Immunol. 1977 Mar;27(3):377–380. [PMC free article] [PubMed] [Google Scholar]
- MEIER R., DESAULLES P. A., SCHAR B. Verschiedenartiger Wirkungstypus bakterieller entzündungserregender Substanzen und anderer Reizstoffe. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1955;224(2):104–122. [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Ramsey W. S. Leucocyte locomotion and chemotaxis. Antibiot Chemother (1971) 1974;19:179–190. doi: 10.1159/000395431. [DOI] [PubMed] [Google Scholar]
- Repo H., Kosunen T. U. Leukocyte migration agarose test for the assessment of human neutrophil chemotaxis. II. Variables in the attraction assay. Scand J Immunol. 1977;6(3):211–218. doi: 10.1111/j.1365-3083.1977.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Repo H. Leukocyte migration agarose test for the assessment of human neutrophil chemotaxis. I. Effects of environmental factors on neutrophil migration under agarose. Scand J Immunol. 1977;6(3):203–209. doi: 10.1111/j.1365-3083.1977.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Wilkinson P. C. A requirement for albumin as carrier for low molecular weight leukocyte chemotactic factors. Exp Cell Res. 1976 Dec;103(2):415–418. doi: 10.1016/0014-4827(76)90277-9. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]