Abstract
Lymphoid cells from human palatine tonsils were identified on tissue sections by membrane or intracellular immunofluorescence (IF) staining. Used in an indirect technique, an anti-IgM and an anti-HTLA (human T lymphocyte antigen) antiserum gave complementary patterns of membrane IF, characteristic of the follicular organization. When serial sections were stained for each of the five classes, immunoglobulin-containing cells from all classes were found. Their relative frequencies were, in decreasing order: IgG: 61.6%; IgA: 17.3%; IgM: 12%; IgE: 7.5%; IgD: 1.6%. These differed from those of the gut-associated lymphoid tissue (IgA greater than IgG greater than IgM) and of the peripheral lymph nodes (IgG greater than IgM greater than IgA), but were close to those of mesenteric nodes. The absence of secretory component in tonsil was an additional difference from gut-associated lymphoid tissue and the relatively high proportion of IgE-containing cells, in the absence of recognized atopy, is another feature in common with mesenteric lymph nodes. Finally, slight differences between these results and those obtained on tonsil cell suspensions suggest that some degree of selection probably occurs during the isolation procedure.
Full text
PDF


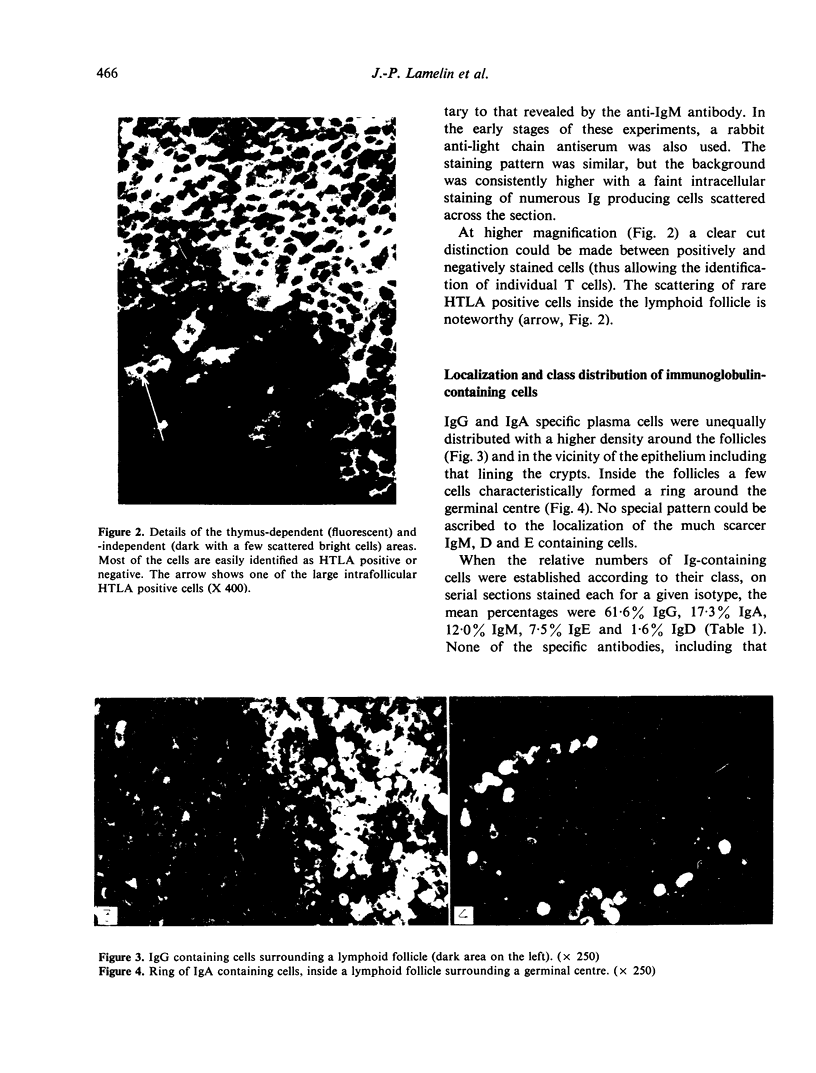



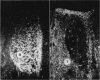
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brochier J., Abou-Hamed Y. A., Gueho J. P., Revillard J. P. Study of human T and B lymphocytes with heterologous antisera. I Preparation, specificity and properties of antisera. Immunology. 1976 Nov;31(5):749–758. [PMC free article] [PubMed] [Google Scholar]
- COOPER M. D., PETERSON R. D., GOOD R. A. DELINEATION OF THE THYMIC AND BURSAL LYMPHOID SYSTEMS IN THE CHICKEN. Nature. 1965 Jan 9;205:143–146. doi: 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Curran R. C., Jones E. L. Immunoglobulin-containing cells in human tonsils as demonstrated by immunohistochemistry. Clin Exp Immunol. 1977 Apr;28(1):103–115. [PMC free article] [PubMed] [Google Scholar]
- Fairfax A. J., Doniach D., Wells C. E. A membrane-associated autoantibody in a case of myasthenia gravis with chronic hepatitis. Clin Exp Immunol. 1976 Sep;25(3):383–388. [PMC free article] [PubMed] [Google Scholar]
- Gergely P., Vanky F., Klein E. Spontaneous and PHA-induced rosetting of human blood, tonsil lymphocytes and MLC blasts with sheep, human and horse erythrocytes. Clin Exp Immunol. 1976 Jul;25(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Weissman I. L. Lymphoid tissue architecture. Experimental analysis of the origin and distribution of T-cells and B-cells. Immunology. 1972 Oct;23(4):465–479. [PMC free article] [PubMed] [Google Scholar]
- MELLORS R. C., KORNGOLD L. THE CELLULAR ORIGIN OF HUMAN IMMUNOGLOBULINS (GAMMA-2, GAMMA-1M, GAMMA-1A). J Exp Med. 1963 Sep 1;118:387–396. doi: 10.1084/jem.118.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarut C., Brochier J., Revillard J. P. Distribution of cells binding erythrocyte-antibody (EA) complexes in human lymphoid populations. Scand J Immunol. 1976;5(3):221–231. doi: 10.1111/j.1365-3083.1976.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Schoorl R., Riviere A. B., Borne A. E., Feltkamp-Vroom T. M. Identification of T and B lymphocytes in human breast cancer with immunohistochemical techniques. Am J Pathol. 1976 Sep;84(3):529–544. [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L. Reactivity of anti-human brain serum with human lymphocytes. Am J Pathol. 1977 Jan;86(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Willson J. K., Jr, Zaremba J. L., Pitts A. M., Pretlow T. G., 2nd A characterization of human tonsillar lymphocytes after separation from other tonsillar cells in an isokinetic gradient of ficoll in tissue culture medium. Am J Pathol. 1976 May;83(2):341–358. [PMC free article] [PubMed] [Google Scholar]
- Willson J. K., Luberoff D. E., Pitts A., Pretlow T. C., 2nd A method for the separation of lymphocytes and plasma cells from the human palatine tonsil using sedimentation in an isokinetic gradient of Ficoll in tissue culture medium. Immunology. 1975 Jan;28(1):161–170. [PMC free article] [PubMed] [Google Scholar]