Abstract
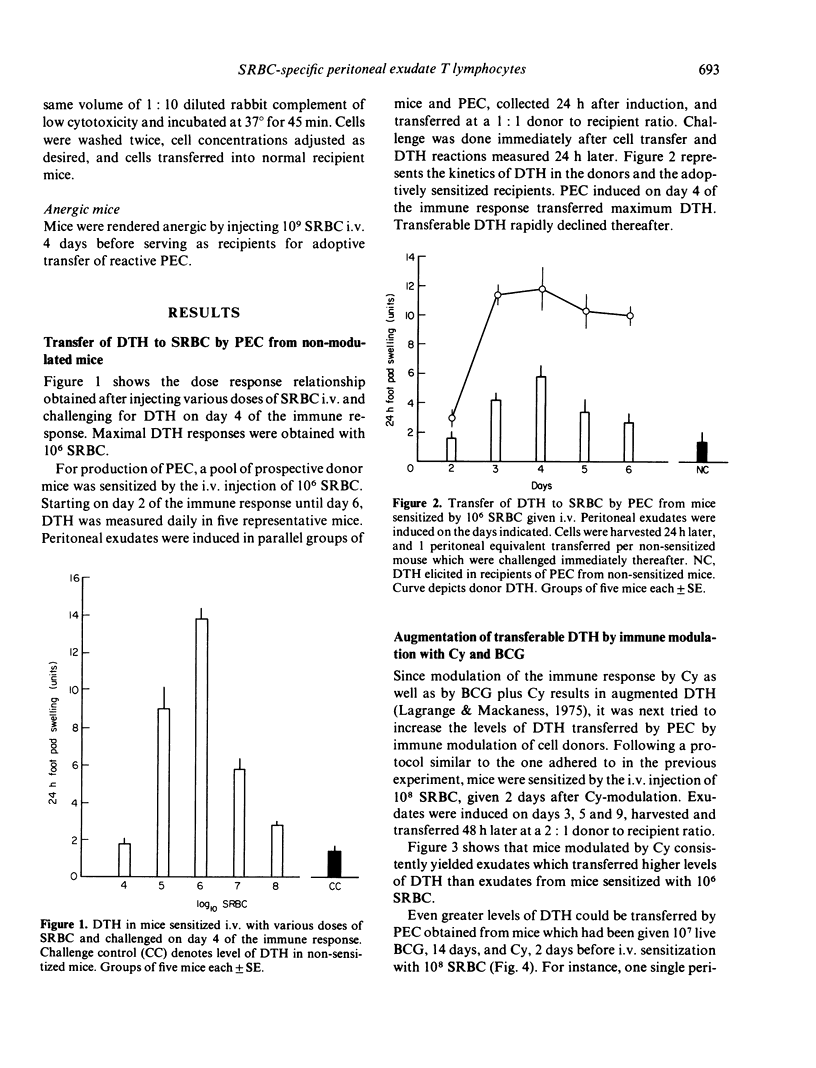
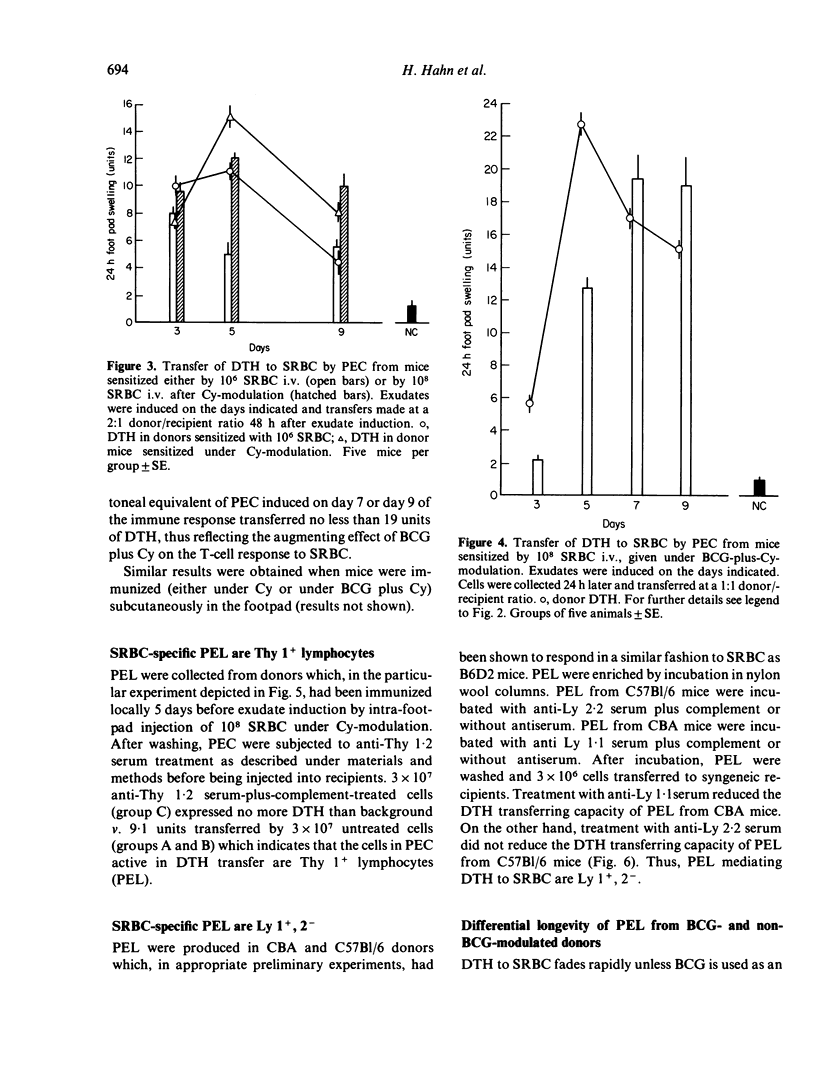
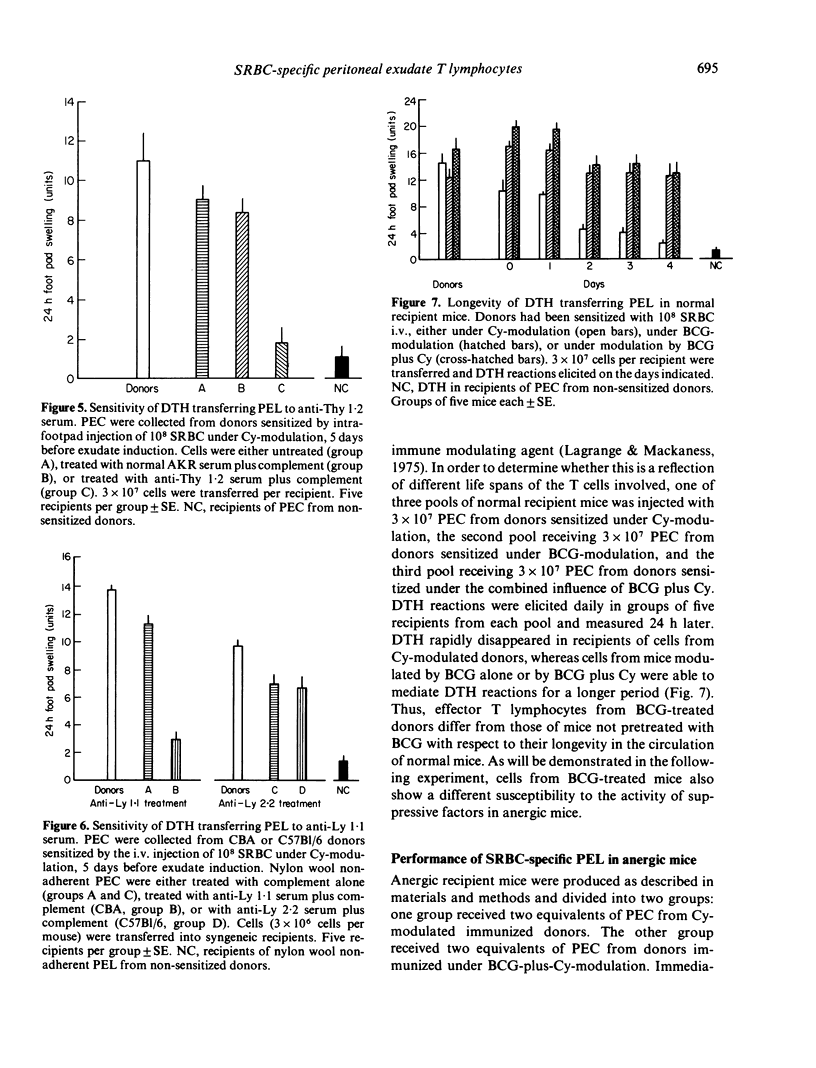
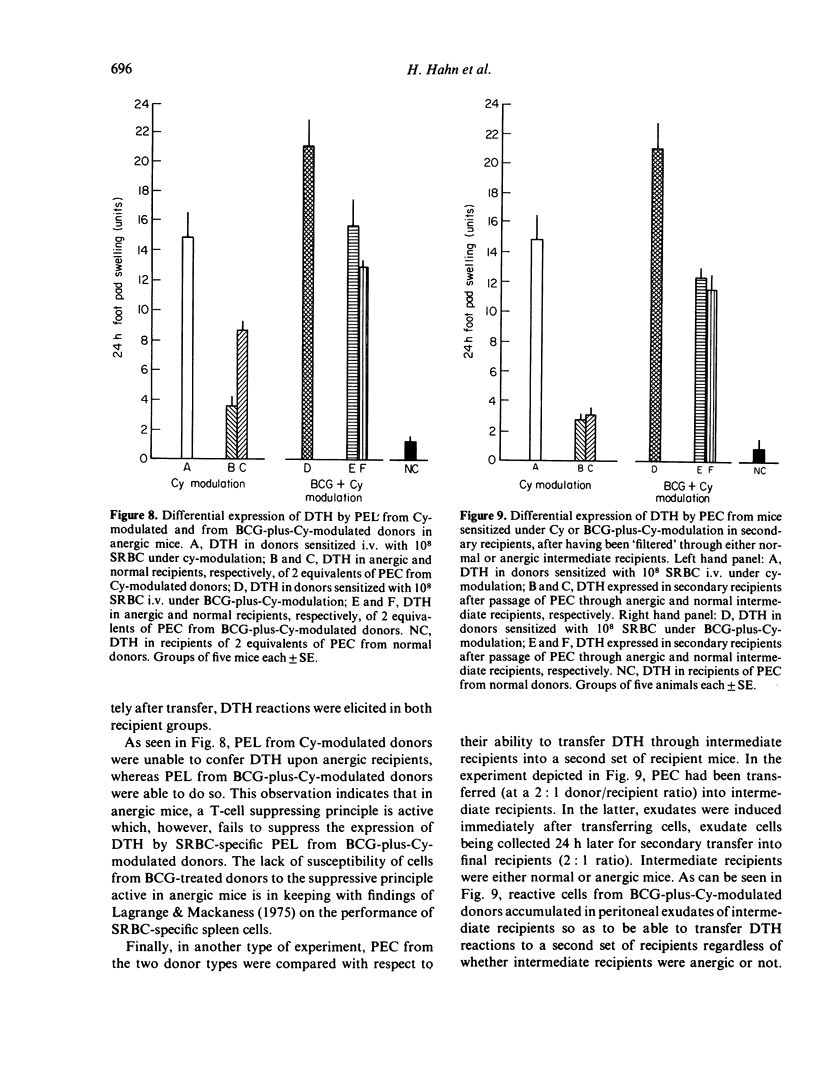
T lymphocytes which mediate DTH reactions to sheep red blood cells (SRBC) in mice enter casein-induced peritoneal exudates from which they can be recovered and assayed in a passive transfer system. Peritoneal exudates need not contain specific antigen for inducement of T-cell immigration. The amount (or biological activity) of DTH-transferring peritoneal exudate lymphocytes is enhanced by the previous use of immune modulating agents, such as cyclophosphamide (Cy) (200 mg/kg 2 days prior to sensitization), or BCG (10(7) live organisms i.v. 14 days prior to sensitization). SRBC-specific peritoneal exudate lymphocytes phenotypically are Thy 1+ and Ly 1+, 2-. In vivo, peritoneal exudate T cells from Cymodulated donors persist in circulation for a short period only and are subject to the suppressive mechanisms acting in anergic mice. Cells from BCG-plus-Cy-modulated donors, on the other hand, persist in circulation for a longer period and appear to be less susceptible to immune suppression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Allwood G. G. Inflammatory lymphoid cells. Cells in immunized lymph nodes that move to sites of inflammation. Immunology. 1972 Mar;22(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Allwood G. G., Mayhew B. Contact sensitivity in the mouse. XI. Movement of T blasts in the draining lymph nodes to sites of inflammation. Immunology. 1973 Sep;25(3):485–494. [PMC free article] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D. The mediator of cellular immunity. 3. Lymphocyte traffic from the blood into the inflamed peritoneal cavity. J Exp Med. 1971 Apr 1;133(4):864–876. doi: 10.1084/jem.133.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B. A stable form of delayed-type hypersensitivity. J Exp Med. 1975 Jan 1;141(1):82–96. doi: 10.1084/jem.141.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- North R. J., Spitalny G. Inflammatory lymphocyte in cell-mediated antibacterial immunity: factors governing the accumulation of mediator T cells in peritoneal exudates. Infect Immun. 1974 Sep;10(3):489–498. doi: 10.1128/iai.10.3.489-498.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell G. D. T cells, T cells recognition structures, and the major histocompatibility complex. Immunol Rev. 1978;38:3–69. doi: 10.1111/j.1600-065x.1978.tb00384.x. [DOI] [PubMed] [Google Scholar]


