Abstract
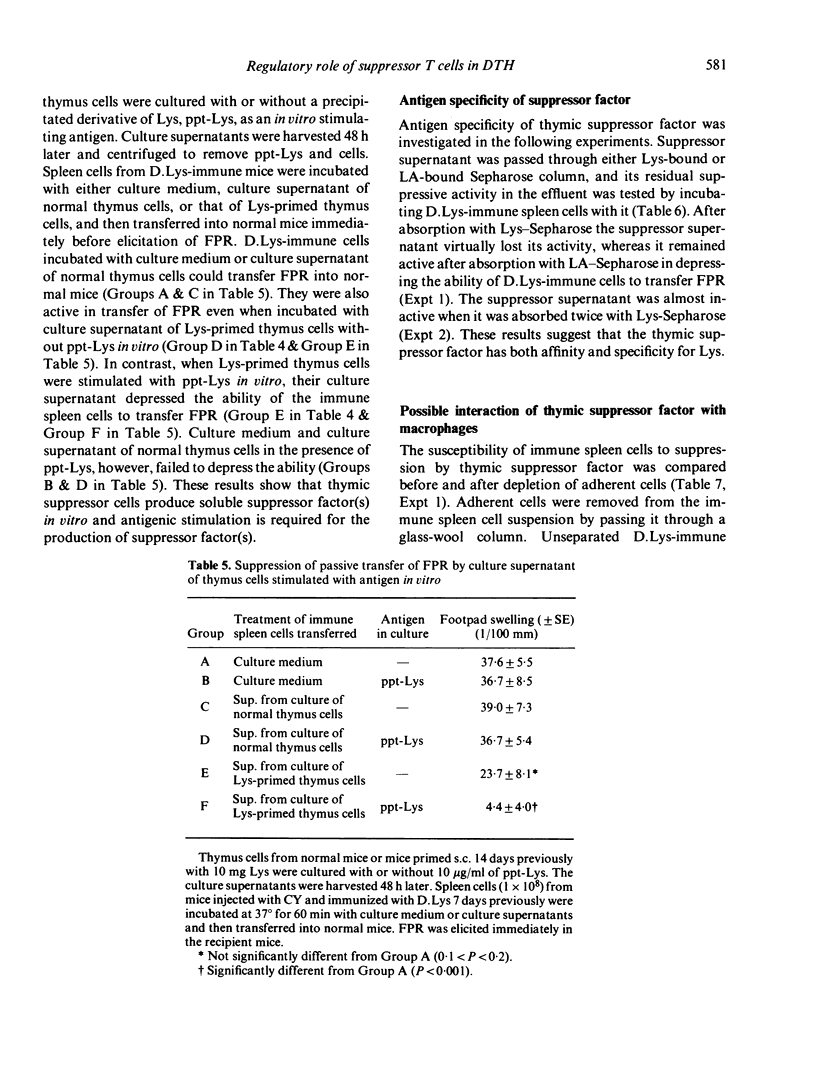
Thymus cells from mice primed s.c. with a high dose (10 mg) of lysozyme (Lys) specifically suppressed delayed footpad reaction (FPR) in mice previously immuned with lipid-conjugated lysozyme (D.Lys), and also suppressed the transfer of FPR by D.Lys-immune spleen cells into normal mice. Furthermore, they inhibited antigen-stimulated DNA synthesis of D.Lys-immune spleen cells in vitro. If the suppressor thymus cells were cultured with Lys in vitro, they produced soluble factor which depressed the ability of D.Lys-immune spleen cells to transfer FPR. Both supernatant of culture without Lys and extract of suppressor thymus cells were inactive in supression of FPR. The suppressor factor was antigen-specific because its suppressive activity was absorbed with Lys but not with an unrelated antigen lactalbumin. The factor failed to depress the ability of D.Lys-immune spleen cells to transfer FPR when the spleen cells were depleted of glass-adherent cells. In addition, incubation of peritoneal exudate cells from normal mice with the factor rendered the cells suppressive for passive transfer of FPR. These results suggest that the suppressor factor depresses the effector function of T cells responsible for FPR possibly via macrophage.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M. Suppression of contact sensitivity by T cells in the mouse. I. Demonstration that suppressor cells act on the effector stage of contact sensitivity; and their induction following in vitro exposure to antigen. Proc R Soc Lond B Biol Sci. 1974 Nov 5;187(1088):329–348. doi: 10.1098/rspb.1974.0078. [DOI] [PubMed] [Google Scholar]
- Coon J., Hunter R. Selective induction of delayed hypersensitivity by a lipid conjugated protein antigen which is localized in thymus dependent lymphoid tissue. J Immunol. 1973 Jan;110(1):183–190. [PubMed] [Google Scholar]
- Feldman J. D. The role of proliferation in delayed hypersensitivity. J Immunol. 1968 Sep;101(3):563–571. [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Kojima A., Egashira Y. Regulatory role of suppressor T cells in the expression of delayed-type hypersensitivity in mice. I. Transient appearance of suppressor T cells for the expression of delayed footpad reaction induced with lipid-conjugated lysozyme. Immunology. 1979 Jul;37(3):569–576. [PMC free article] [PubMed] [Google Scholar]
- Kojima A., Sugimoto M., Egashira Y. Immunogenicity of lysozyme derivatives lipid-conjugated to various degrees in mice treated with and without cyclophosphamide: dissociation of delayed-type hypersensitivity and helper function. Jpn J Med Sci Biol. 1976 Dec;29(6):323–333. doi: 10.7883/yoken1952.29.323. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Chan-Liew W. L. Regulation of delayed-type hypersensitivity. II. Specific suppressor factor for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):168–171. doi: 10.1002/eji.1830080305. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. I. T suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1977 Oct;7(10):714–718. doi: 10.1002/eji.1830071013. [DOI] [PubMed] [Google Scholar]
- Moorhead J. W. Soluble factors in tolerance and contact sensitivity to 2,4-dinitrofluorobenzene in mice. I. Suppression of contact sensitivity by soluble suppressor factor released in vitro by lymph node cell populations containing specific suppressor cells. J Immunol. 1977 Jul;119(1):315–321. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFA in mice. VIII. Identification of distinct T cell subpopulations that mediate in vivo and in vitro manifestations of delayed hypersensitivity. J Immunol. 1978 Jan;120(1):137–144. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFB in mice. VI. Inhibition of afferent sensitivity by suppressor T cells in adoptive tolerance. J Immunol. 1976 Sep;117(3):802–806. [PubMed] [Google Scholar]
- Phanupak P., Moorhead J. W., Claman H. N. Tolerance and contact sensitivity to DNFB in mice. 3. Transfer of tolerance with "suppressor T cells". J Immunol. 1974 Oct;113(4):1230–1236. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Bretscher P. A., Parish C. R. Regulation of the immune response. I. Suppression of delayed-type hypersensitivity by T cells from mice expressing humoral immunity. Eur J Immunol. 1976 Oct;6(10):674–679. doi: 10.1002/eji.1830061003. [DOI] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. Depression of the T cell phenomenon of contact sensitivity by T cells from unresponsive mice. Nature. 1973 Jul 27;244(5413):227–228. doi: 10.1038/244227a0. [DOI] [PubMed] [Google Scholar]


