Abstract
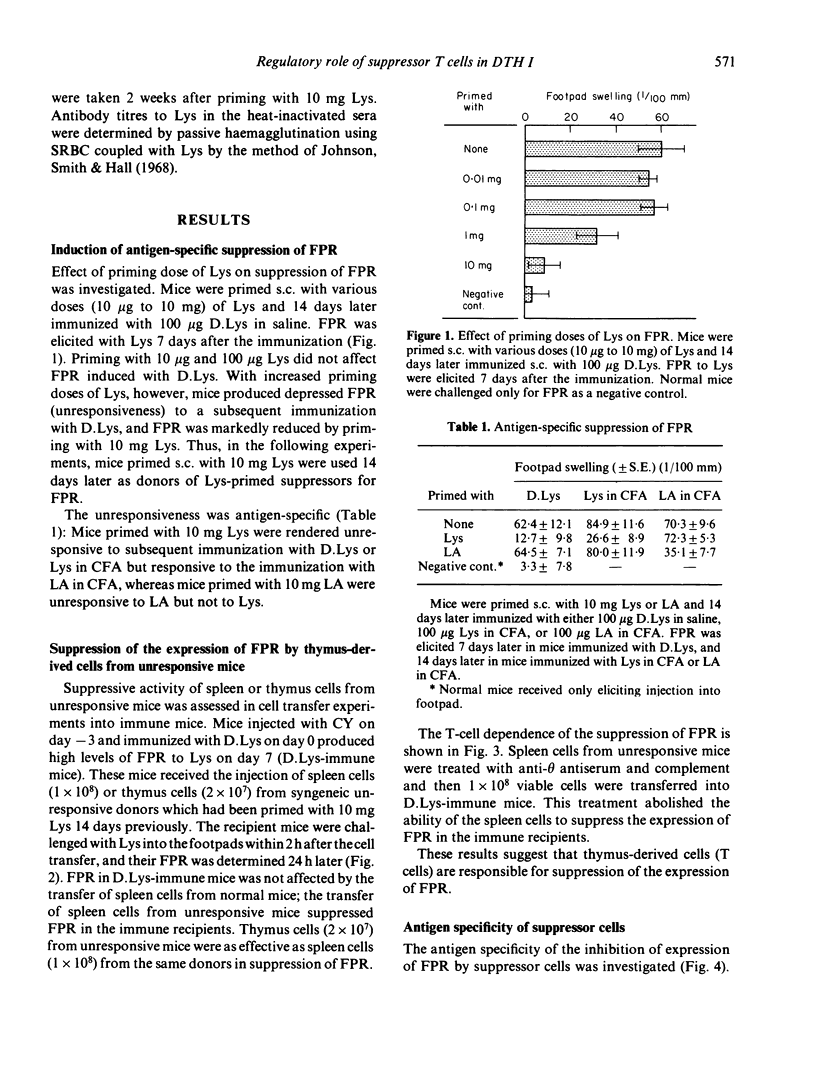
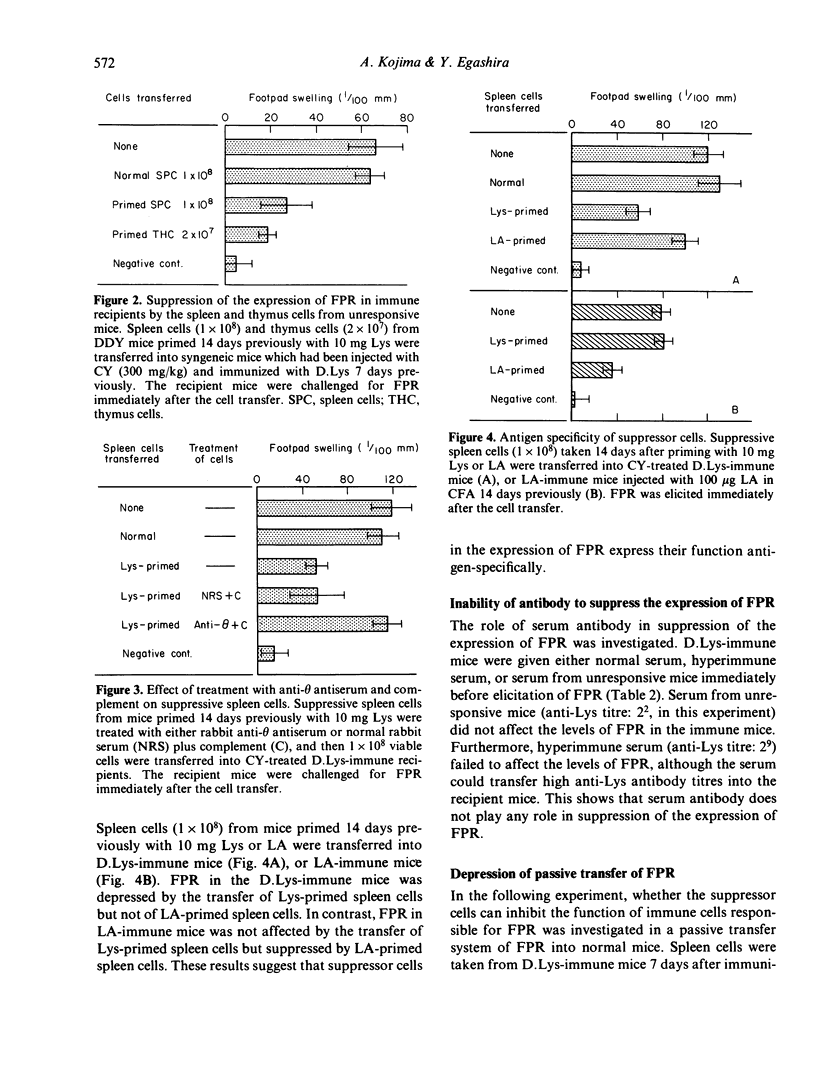
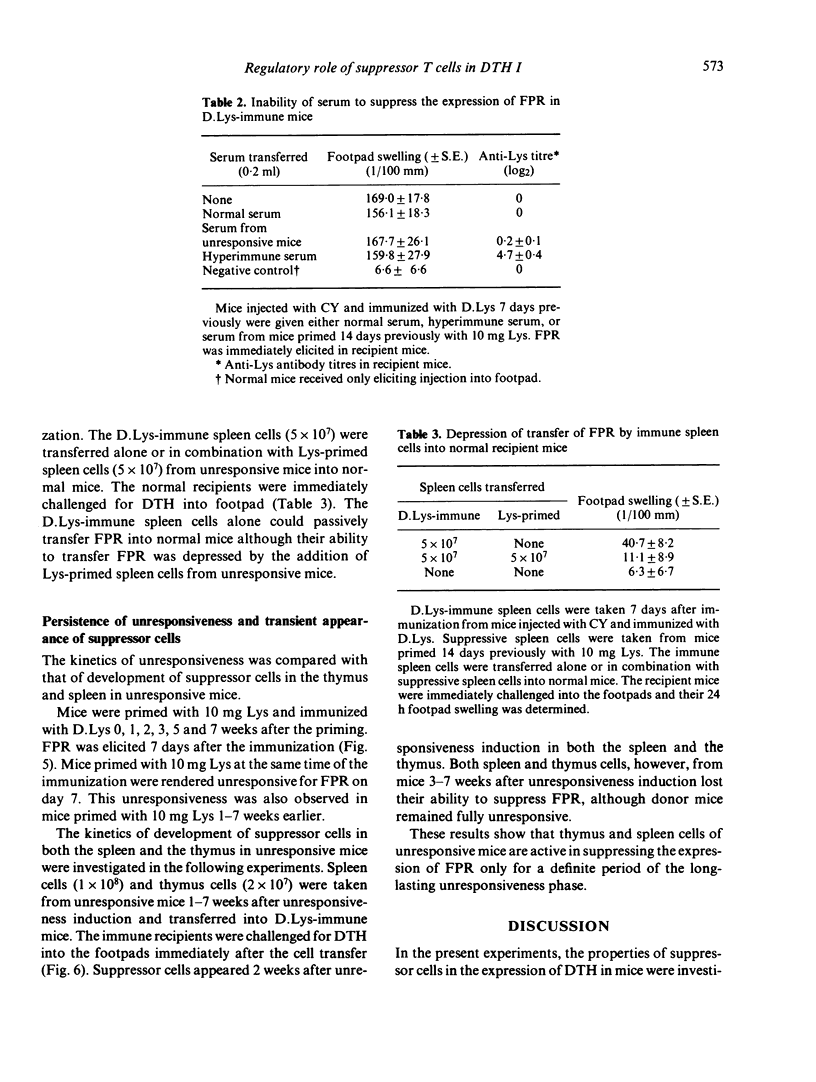
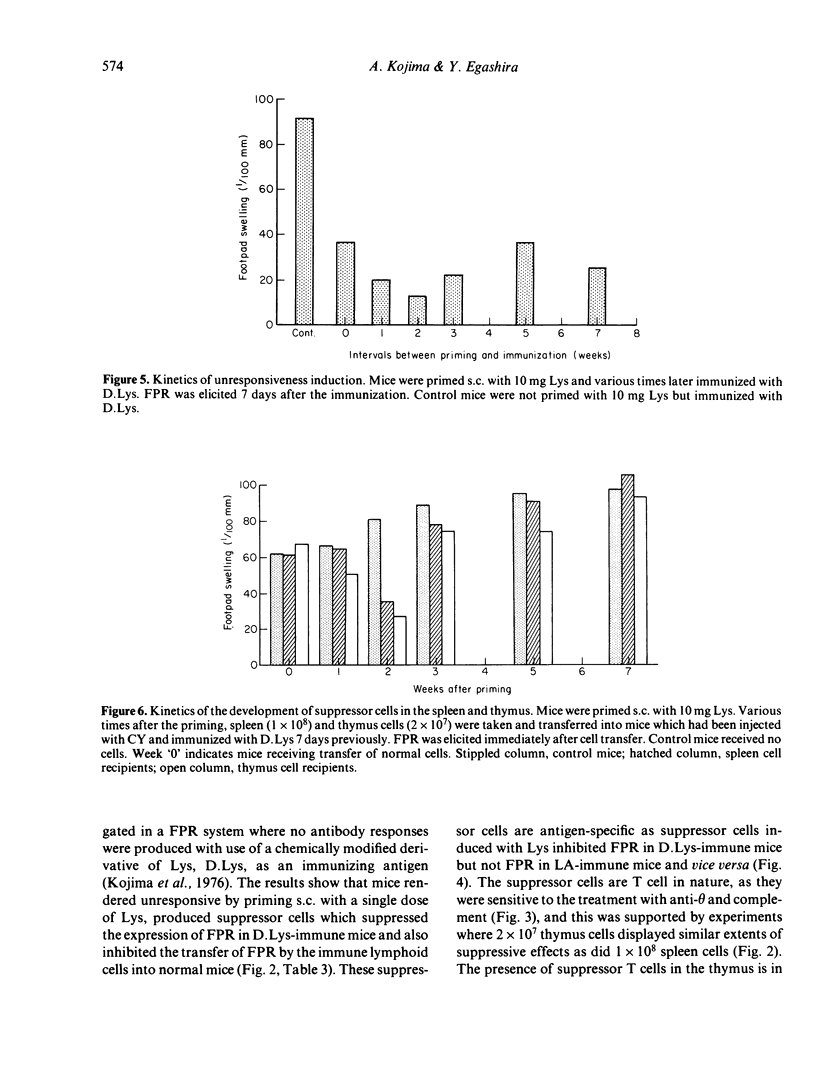
Delayed footpad reaction (FPR) to lysozyme (Lys) in mice was induced without antibody responses by lipid-conjugated lysozyme (D.Lys). This FPR was suppressed by priming s.c. with a high dose (10 mg) of Lys 2 weeks previously (unresponsiveness). Spleen cells from the unresponsive mice suppressed antigen-specifically FPR in mice previously immunized with D.Lys, and also suppressed passive transfer of FPR by D.Lys-immune lymphoid cells into normal mice. The suppressive activity of the spleen cells was abolished by treatment with anti-phi anti-serum and complement. The suppressor cells occurred also in the thymus of unresponsive mice. Unresponsiveness was induced in mice immediately after priming with Lys and persisted at least up to 7 weeks after the induction. In contrast, suppressor cells appeared only 2 weeks after induction of unresponsiveness in both the spleen and the thymus but were no longer detectable 3-7 weeks later, although donor mice remained fully unresponsive. These results suggest that antigen-specific suppressor T cells are involved in the regulation of the expression of FPR only for a definite period of time in unresponsive mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M. Suppression of contact sensitivity by T cells in the mouse. I. Demonstration that suppressor cells act on the effector stage of contact sensitivity; and their induction following in vitro exposure to antigen. Proc R Soc Lond B Biol Sci. 1974 Nov 5;187(1088):329–348. doi: 10.1098/rspb.1974.0078. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Moorhead J. W. Tolerance: two pathways of negative immunoregulation in contact sensitivity to DNFB. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):105–111. doi: 10.1101/sqb.1977.041.01.014. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Coon J., Hunter R. Selective induction of delayed hypersensitivity by a lipid conjugated protein antigen which is localized in thymus dependent lymphoid tissue. J Immunol. 1973 Jan;110(1):183–190. [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. Effect of cyclophosphamide on delayed hypersensitivity to Staphylococcus aureus in mice. Immunology. 1977 Nov;33(5):767–776. [PMC free article] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity. III. Effect of cyclophosphamide on the suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):172–176. doi: 10.1002/eji.1830080306. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H. Role of the thymus in tolerance. X. "Suppressor" activity of antigen-stimulated rat thymocytes transferred to normal recipients. J Immunol. 1973 May;110(5):1290–1299. [PubMed] [Google Scholar]
- Johnson H. M., Smith B. G., Hall H. E. Carbodiimide hemagglutination. A study of some of the variables of the coupling reaction. Int Arch Allergy Appl Immunol. 1968;33(5):511–520. [PubMed] [Google Scholar]
- Kojima A., Sugimoto M., Egashira Y. Immunogenicity of lysozyme derivatives lipid-conjugated to various degrees in mice treated with and without cyclophosphamide: dissociation of delayed-type hypersensitivity and helper function. Jpn J Med Sci Biol. 1976 Dec;29(6):323–333. doi: 10.7883/yoken1952.29.323. [DOI] [PubMed] [Google Scholar]
- Kojima A., Tamura S. I., Egashira Y. Regulatory role of suppressor T cells in the expression of delayed-type hypersensitivity in mice. II. Soluble factor from thymic suppressor cells stimulated with antigen in vitro and its possible interaction with macrophages. Immunology. 1979 Jul;37(3):577–585. [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. I. T suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1977 Oct;7(10):714–718. doi: 10.1002/eji.1830071013. [DOI] [PubMed] [Google Scholar]
- Loblay R. H., Pritchand-Briscoe H., Basten A. Suppressor T-cell memory. Nature. 1978 Apr 13;272(5654):620–622. doi: 10.1038/272620a0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. The induction of hapten-specific T cell tolerance using hapten-modified lymphoid membranes. II. Relative roles of suppressor T cells and clone inhibition in the tolerant state. Eur J Immunol. 1977 Mar;7(3):165–170. doi: 10.1002/eji.1830070310. [DOI] [PubMed] [Google Scholar]
- Phanupak P., Moorhead J. W., Claman H. N. Tolerance and contact sensitivity to DNFB in mice. 3. Transfer of tolerance with "suppressor T cells". J Immunol. 1974 Oct;113(4):1230–1236. [PubMed] [Google Scholar]
- Polak L., Turk J. L. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974 Jun 14;249(458):654–656. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Bretscher P. A., Parish C. R. Regulation of the immune response. I. Suppression of delayed-type hypersensitivity by T cells from mice expressing humoral immunity. Eur J Immunol. 1976 Oct;6(10):674–679. doi: 10.1002/eji.1830061003. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Kojima A., Yaginuma K., Egashira Y. Cell-mediated and humoral immunity in mice: cross reaction between lysozyme and S-carboxymethylated lysozyme studied by a modified footpad test. Jpn J Med Sci Biol. 1975 Feb;28(1):23–35. doi: 10.7883/yoken1952.28.23. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Parker D., Poulter L. W. Functional aspects of the selective depletion of lymphoid tissue by cyclophosphamide. Immunology. 1972 Oct;23(4):493–501. [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. Depression of the T cell phenomenon of contact sensitivity by T cells from unresponsive mice. Nature. 1973 Jul 27;244(5413):227–228. doi: 10.1038/244227a0. [DOI] [PubMed] [Google Scholar]


