Abstract
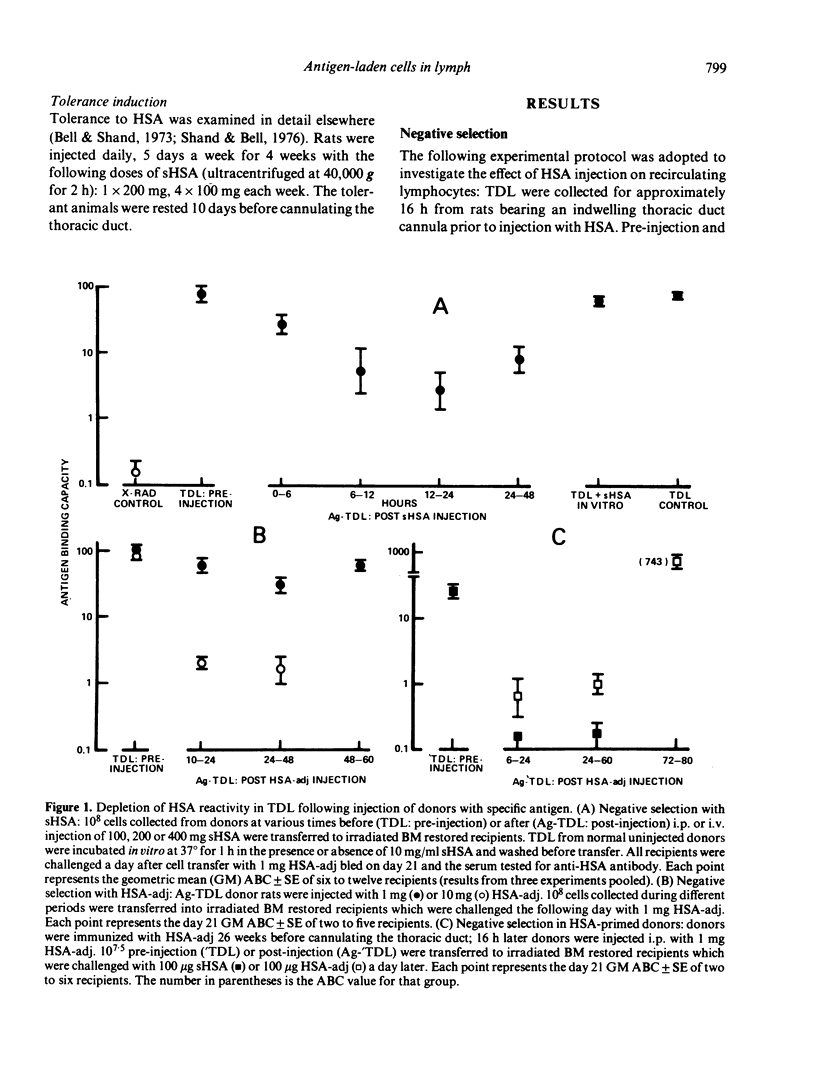
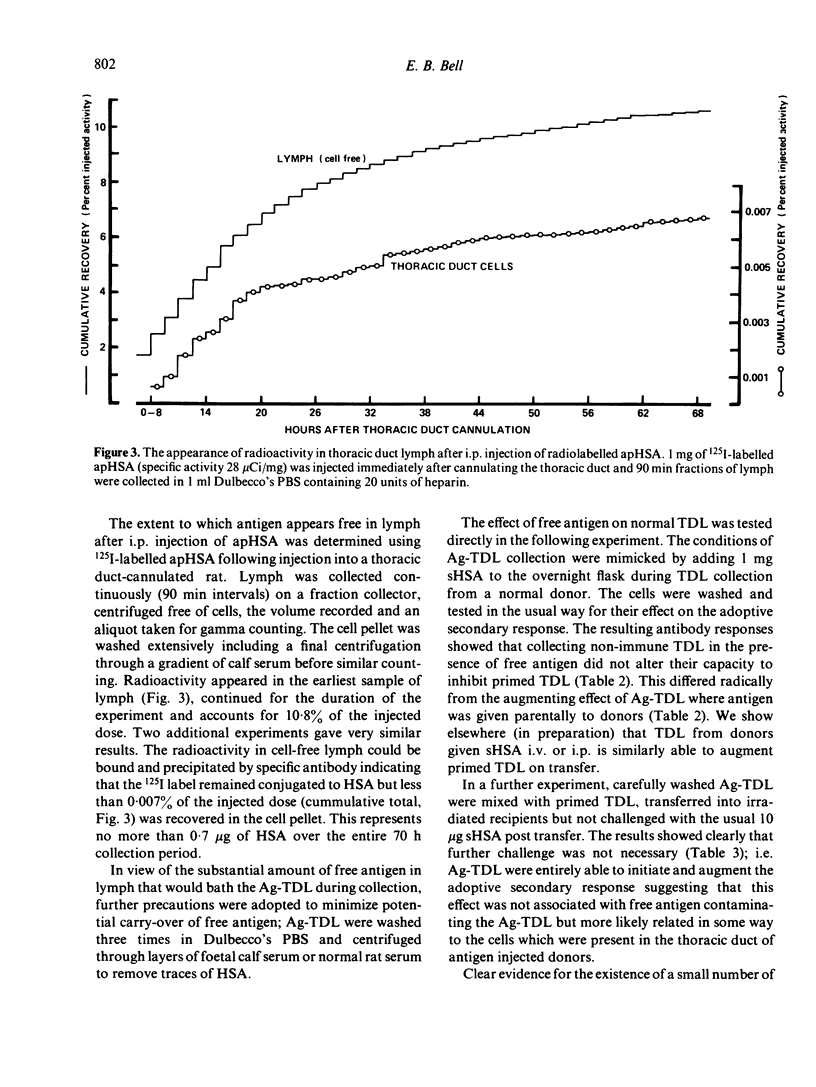
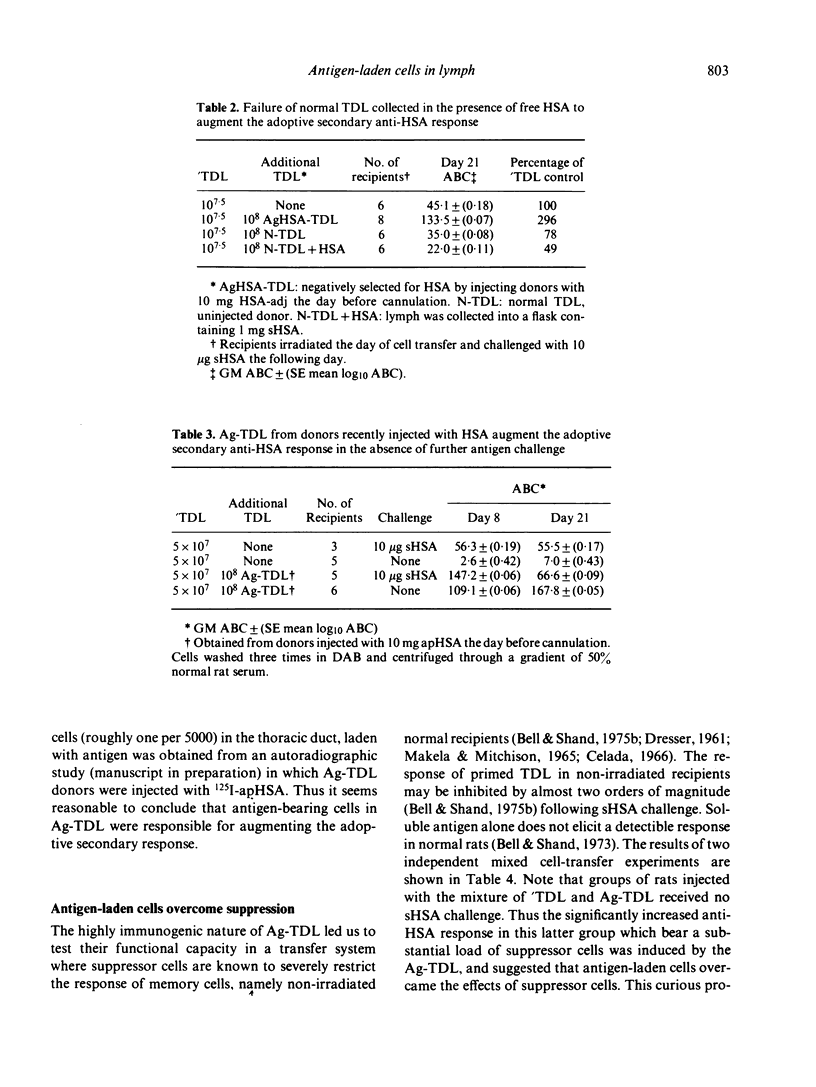
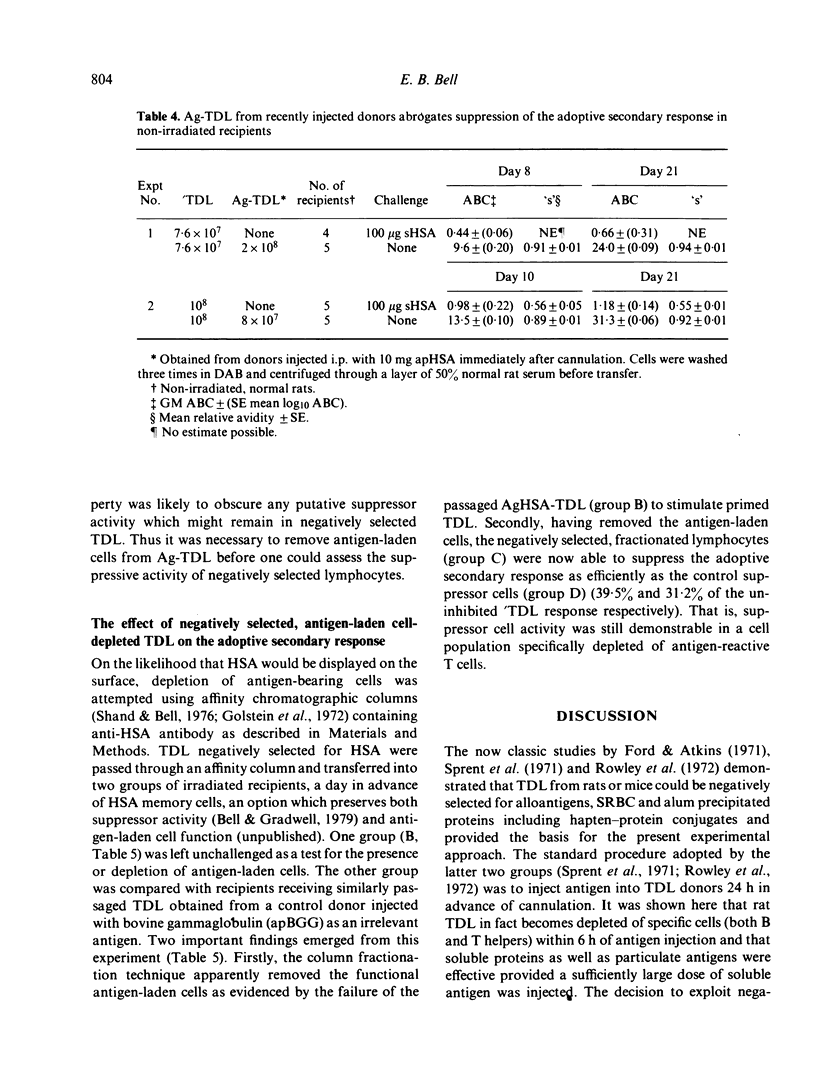
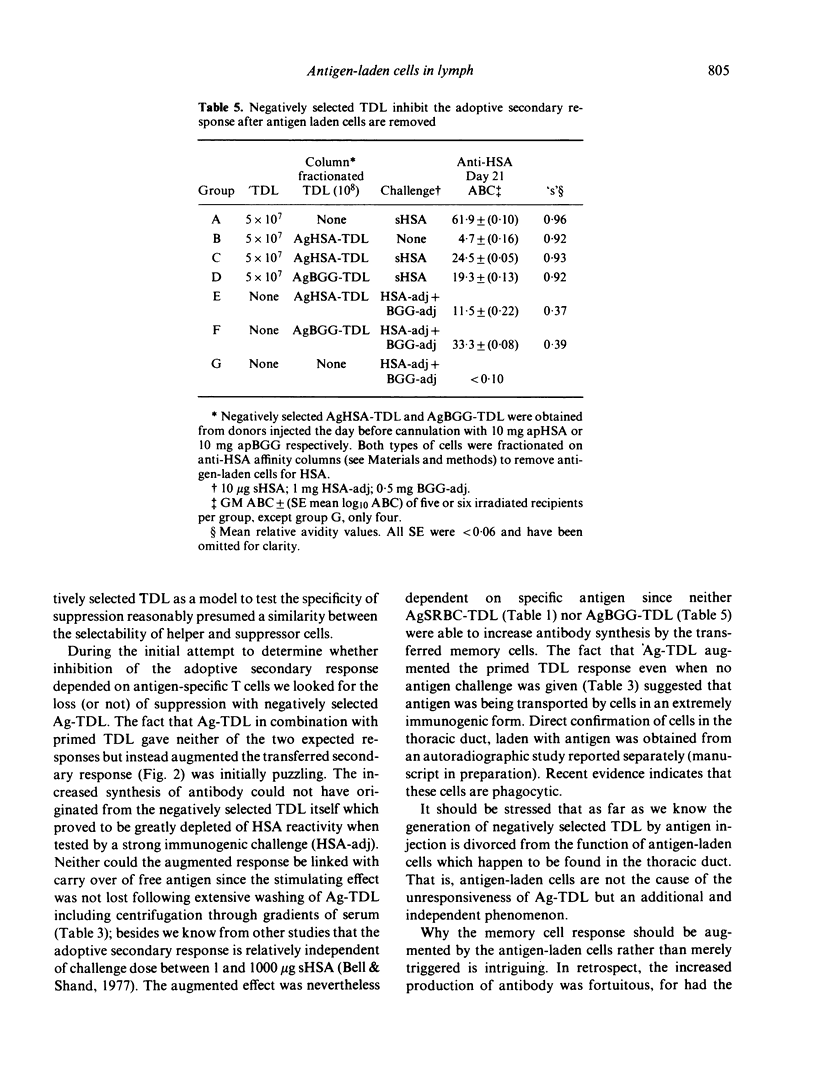
T cells from thoracic duct lymph of donor rats suppress the adoptive secondary response to human serum albumin (HSA). The original aim of the present investigation was to determine whether these non-immune cells have antigen-specific receptors. Thoracic duct lymphocytes (TDL) were depleted in vivo of antigen-specific T cells (negatively selected) by acutely injecting non-immune donors with HSA at the time of thoracic duct cannulation. Negatively selected TDL were mixed with memory cells (primed TDL from previously immunized donors) and transferred into irradiated recipients to assess whether the suppressive potential had disappeared. Paradoxically, the addition of negatively selected TDL (which were unresponsive to HSA) augmented the adoptive secondary anti-HSA response. Further study showed that the augmented response was mediated by a very small number of cells (∼ 1 in 5000) laden with antigen that appeared in lymph of non-immune donors following HSA injection. These antigen-bearing cells were highly immunogenic and furthermore could overcome the effects of T suppressor cells in vivo. Once antigen laden cells were removed from lymph (by affinity chromatography), however, negatively selected TDL were found to inhibit the adoptive secondary response suggesting that either suppression in this model is non-specific or that antigen-specific suppressor cells are not selected out of the recirculating pool by antigen.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Johnson P. T cell-dependent suppression of an anti-hapten antibody response. Transplant Rev. 1975;26:130–169. doi: 10.1111/j.1600-065x.1975.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Gradwell S. Studies on the mechanism of suppression by T cells in an adoptive secondary response. Cell Immunol. 1979 Jan;42(1):113–123. doi: 10.1016/0008-8749(79)90226-0. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Shand F. L. Cellular events in protein tolerant inbred rats. I. The fate of thoracic duct lymphocytes and memory cells during tolerance induction to human serum albumin. Eur J Immunol. 1973 May;3(5):259–267. doi: 10.1002/eji.1830030503. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Shand F. L. Changes in lymphocyte recirculation and liberation of the adoptive memory response from cellular regulation in irradiated recipients. Eur J Immunol. 1975 Jan;5(1):1–7. doi: 10.1002/eji.1830050102. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Shand F. L. Persisting T cells in rats tolerant of human serum albumin. The significance of tolerant and nonimmune T cells which preferentially restrict high affinity antibody synthesis. Eur J Immunol. 1976 Jul;5(7):481–486. doi: 10.1002/eji.1830050710. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Shand F. L. The adoptive secondary response to human serum albumin under conditions of high antigen pressure. The response of high and low avidity B cell subsets. Immunology. 1977 Oct;33(4):469–476. [PMC free article] [PubMed] [Google Scholar]
- Calderon J., Kiely J. M., Lefko J. L., Unanue E. R. The modulation of lymphocyte functions by molecules secreted by macrophages. I. Description and partial biochemical analysis. J Exp Med. 1975 Jul 1;142(1):151–164. doi: 10.1084/jem.142.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F., Schmidt D., Strom R. Determination of avidity of anti-albumin antibodies in the mouse. Influence of the number of cells transferred on the quality of the secondary adoptive response. Immunology. 1969 Aug;17(2):189–198. [PMC free article] [PubMed] [Google Scholar]
- DRESSER D. W. A study of the adoptive secondary response to a protein antigen in mice. Proc R Soc Lond B Biol Sci. 1961 Jul 25;154:398–417. doi: 10.1098/rspb.1961.0039. [DOI] [PubMed] [Google Scholar]
- Elson C. J., Taylor R. B. The suppressive effect of carrier priming on the response to a hapten-carrier conjugate. Eur J Immunol. 1974 Oct;4(10):682–687. doi: 10.1002/eji.1830041009. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. I. The requirement for macrophages in helper cell induction and characteristics of the macrophage-T cell interaction. Cell Immunol. 1975 Oct;19(2):356–367. doi: 10.1016/0008-8749(75)90217-8. [DOI] [PubMed] [Google Scholar]
- Feldbush T. L. Inhibition of adoptive secondary responses by lymphoid cell populations. Cell Immunol. 1976 Jun 1;24(1):132–145. doi: 10.1016/0008-8749(76)90139-8. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Atkins R. C. Specific unresponsiveness of recirculating lymphocytes ater exposure to histocompatibility antigen in F 1 hybrid rats. Nat New Biol. 1971 Dec 8;234(49):178–180. doi: 10.1038/newbio234178a0. [DOI] [PubMed] [Google Scholar]
- Ford W. L. The recruitment of recirculating lymphocytes in the antigenically stimulated spleen. Specific and non-specific consequences of initiating a secondary antibody response. Clin Exp Immunol. 1972 Oct;12(2):243–254. [PMC free article] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E. A. Cells mediating specific in vitro cytotoxicity. II. Probable autonomy of thymus-processed lymphocytes (T cells) for the killing of allogeneic target cells. J Exp Med. 1972 Apr 1;135(4):890–906. doi: 10.1084/jem.135.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Adachi T. Generation of specific helper cells and suppressor cells in vitro for the IgE and IgG antibody responses. J Immunol. 1976 Jul;117(1):40–47. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Mäkelä O., Mitchison N. A. The role of cell number and source in adoptive immunity. Immunology. 1965 Jun;8(6):539–548. [PMC free article] [PubMed] [Google Scholar]
- Niederhuber J. E. The role of I region gene products in macrophage - T lymphocyte interaction. Immunol Rev. 1978;40:28–52. doi: 10.1111/j.1600-065x.1978.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Okumura K., Takemori T., Tokuhisa T., Tada T. Specific enrichment of the suppressor T cell bearing I-J determinants: parallel functional and serological characterizations. J Exp Med. 1977 Nov 1;146(5):1234–1245. doi: 10.1084/jem.146.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Germain R. N. Antigen-specific T cell-mediated suppression. IV. Role of macrophages in generation of L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT)-specific suppressor T cells in responder mouse strains. J Immunol. 1978 Oct;121(4):1306–1314. [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. The participation of macrophages and macrophage cell lines in the activation of T lymphocytes by mitogens. Immunol Rev. 1978;40:102–135. doi: 10.1111/j.1600-065x.1978.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Gowans J. L., Atkins R. C., Ford W. L., Smith M. E. The specific selection of recirculating lymphocytes by antigen in normal and preimmunized rats. J Exp Med. 1972 Sep 1;136(3):499–513. doi: 10.1084/jem.136.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Paul W. E. Interaction between antigen-presenting cells and primed T lymphocytes: an assessment of Ir gene expression in the antigen-presenting cell. Immunol Rev. 1978;40:153–180. doi: 10.1111/j.1600-065x.1978.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Shand F. L., Bell E. B. Cellular events in protein-tolerant inbred rats. III. Antigen-reactive B cells in rats tolerant to human serum albumin. Cell Immunol. 1976 Jan;21(1):20–30. doi: 10.1016/0008-8749(76)90323-3. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Sprent J. Restricted helper function of F1 hybrid T cells positively selected to heterologous erythrocytes in irradiated parental strain mice. I. Failure to collaborate with B cells of the opposite parental strain not associated with active suppression. J Exp Med. 1978 Apr 1;147(4):1142–1158. doi: 10.1084/jem.147.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Takemori T. Selective roles of thymus-derived lymphocytes in the antibody response. I. Differential suppressive effect of carrier-primed T cells on hapten-specific IgM and IgG antibody responses. J Exp Med. 1974 Jul 1;140(1):239–252. doi: 10.1084/jem.140.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Miller J. F. Enrichment of specific suppressor T cells and characterization of their surface markers. J Exp Med. 1977 Nov 1;146(5):1450–1454. doi: 10.1084/jem.146.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Weiss A., Fitch F. W. Suppression of the plaque-forming cell response by macrophages present in the normal rat spleen. J Immunol. 1978 Feb;120(2):357–359. [PubMed] [Google Scholar]
- Wigzell H. Specific fractionation of immunocompetent cells. Transplant Rev. 1970;5:76–104. doi: 10.1111/j.1600-065x.1970.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hamaoka T., Yoshizawa M., Kuroki M., Kitagawa M. Regulatory functions of hapten-reactive helper and suppressor T lymphocytes. I. Detection and characterization of hapten-reactive suppressor T-cell activity in mice immunized with hapten-isologous protein conjugate. J Exp Med. 1977 Jul 1;146(1):74–90. doi: 10.1084/jem.146.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]


